Fajans Rules Explaining exceptions to electronegativity calculations Exceptions









- Slides: 13

Fajan’s Rules Explaining exceptions to electronegativity calculations

Exceptions to the rules • Exceptions to chemical rules drive us crazy. • Sometimes bonds can have characteristics that we would not expect. • Fajan’s rules help us explain some of these exceptions rationally.

Fajan’s rules deal with The size of the atomic radius

Fajan’s rules deal with • The amount of charge an atom has on it. A +1 or -1 is considered low while a +4 or -4 is considered a high charge.

Fajan’s rules deal with The ability of something to behave as a nonpolar covalent compound, a polar covalent compound, or an ionic compound.

Fajan’s rules deal with It discusses how an ionic bond can have covalent character and how a covalent bond can have ionic character by distorting the electron cloud surrounding the ion.

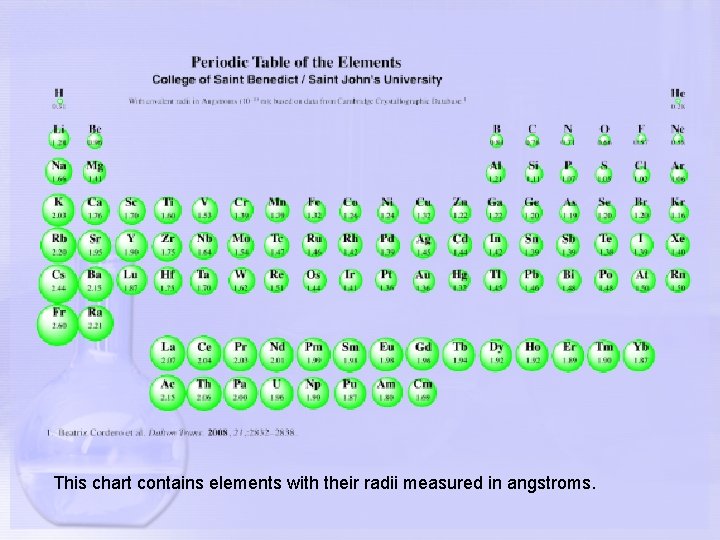
This chart contains elements with their radii measured in angstroms.

Observations: Not all ionic compounds have high melting points. Some covalently bonded compounds have higher than expected boiling points due to dipoles in their structure. Reason: in many substances the bonding is not 100% ionic or covalent

Ideal ionic compoundcompletely separate, spherical ions electron densities are apart from each other. However, if the positive ion has a high charge density it can distort the negative ion by attracting the outer shell electrons to give an area of electron density between the two species. . . a bit like a covalent bond. This is what is happening in the Lil bond on your homework.

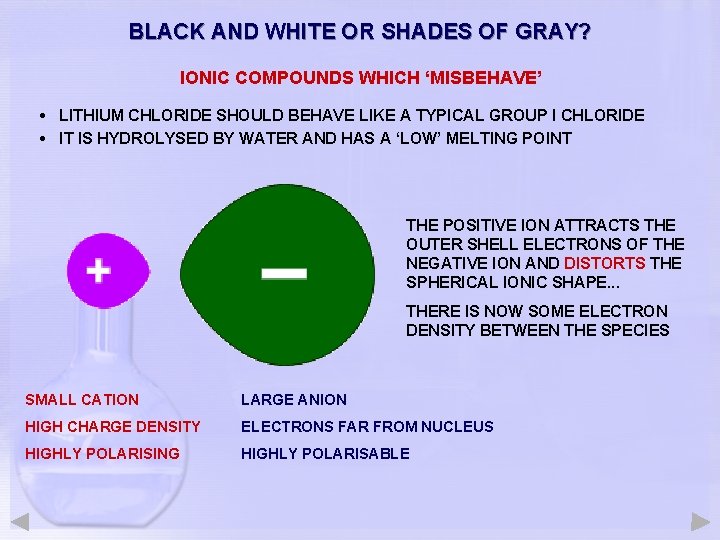
BLACK AND WHITE OR SHADES OF GRAY? IONIC COMPOUNDS WHICH ‘MISBEHAVE’ • LITHIUM CHLORIDE SHOULD BEHAVE LIKE A TYPICAL GROUP I CHLORIDE • IT IS HYDROLYSED BY WATER AND HAS A ‘LOW’ MELTING POINT THE POSITIVE ION ATTRACTS THE OUTER SHELL ELECTRONS OF THE NEGATIVE ION AND DISTORTS THE SPHERICAL IONIC SHAPE. . . THERE IS NOW SOME ELECTRON DENSITY BETWEEN THE SPECIES SMALL CATION LARGE ANION HIGH CHARGE DENSITY ELECTRONS FAR FROM NUCLEUS HIGHLY POLARISING HIGHLY POLARISABLE

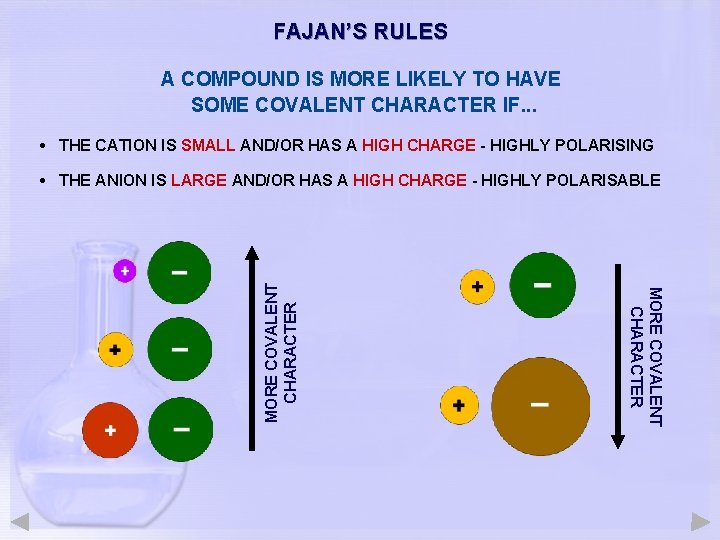
FAJAN’S RULES A COMPOUND IS MORE LIKELY TO HAVE SOME COVALENT CHARACTER IF. . . • THE CATION IS SMALL AND/OR HAS A HIGH CHARGE - HIGHLY POLARISING MORE COVALENT CHARACTER • THE ANION IS LARGE AND/OR HAS A HIGH CHARGE - HIGHLY POLARISABLE

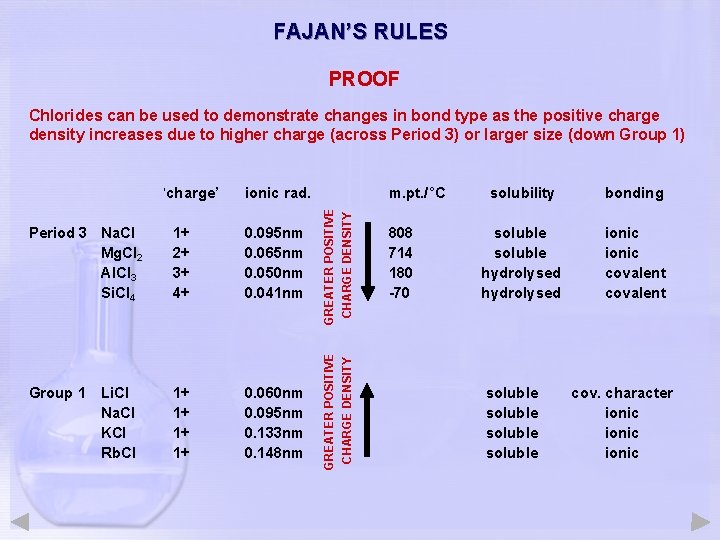
FAJAN’S RULES PROOF Chlorides can be used to demonstrate changes in bond type as the positive charge density increases due to higher charge (across Period 3) or larger size (down Group 1) Period 3 Na. Cl Mg. Cl 2 Al. Cl 3 Si. Cl 4 1+ 2+ 3+ 4+ 0. 095 nm 0. 065 nm 0. 050 nm 0. 041 nm Group 1 Li. Cl Na. Cl KCl Rb. Cl 1+ 1+ 0. 060 nm 0. 095 nm 0. 133 nm 0. 148 nm m. pt. /°C GREATER POSITIVE CHARGE DENSITY ionic rad. GREATER POSITIVE CHARGE DENSITY ‘charge’ 808 714 180 -70 solubility bonding soluble hydrolysed ionic covalent soluble cov. character ionic

Fajan’s rules 1. Cation, covalent character increases with increasing anion size. F<Cl<Br<I. 2. Anion, covalent character increases with decreasing cation size. K<Na<Li. 3. The covalent character increases with increasing charge on either ion. 4. Covalent character is greater for cations with non-noble gas electronic configurations.