American Health Information Community Personalized Health Care Workgroup



















- Slides: 20

American Health Information Community Personalized Health Care Workgroup Newborn Screening Draft Recommendations Kristin Brinner Workgroup Coordinator February 6, 2008

Personalized Health Care (PHC) Workgroup Member List • Co-Chairs: – – • Partners Health. Care American Academy of Family Physicians Staff Co-Chair: – • John Glaser Douglas Henley Gregory Downing Office of the Secretary, HHS Members: – – – – – – Carolyn Clancy Beryl Crossley Paul Cusenza Andrea Ferreira-Gonzalez Becky Fisher Felix Frueh Emory Fry Alan Guttmacher Kathy Hudson Betsy Humphreys Charles Kennedy Joel Kupersmith Stephen Matteson Deven Mc. Graw Amy Mc. Guire Mark Rothstein Steve Teutsch Janet Warrington Andrew Wiesenthal Dennis Williams Marc Williams Agency for Healthcare Research and Quality American Clinical Laboratory Association, Quest Entrepreneur and Consultant Virginia Commonwealth University Patient Advocate Food and Drug Administration Department of Defense National Institutes of Health/NHGRI Genetics and Public Policy Center National Institutes of Health/NLM Well. Point Department of Veterans Affairs Pfizer National Partnership for Women and Families Baylor College of Medicine University of Louisville Merck Affymetrix Inc. Permanente Federation Health Resources and Services Administration Intermountain Healthcare 2

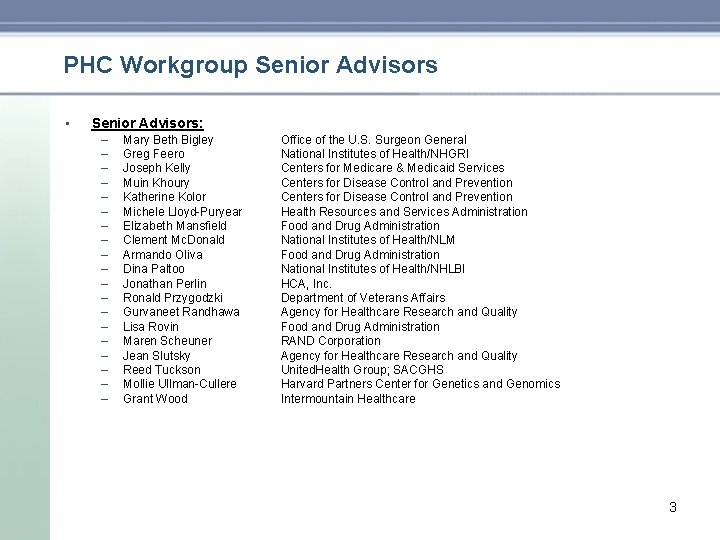
PHC Workgroup Senior Advisors • Senior Advisors: – – – – – Mary Beth Bigley Greg Feero Joseph Kelly Muin Khoury Katherine Kolor Michele Lloyd-Puryear Elizabeth Mansfield Clement Mc. Donald Armando Oliva Dina Paltoo Jonathan Perlin Ronald Przygodzki Gurvaneet Randhawa Lisa Rovin Maren Scheuner Jean Slutsky Reed Tuckson Mollie Ullman-Cullere Grant Wood Office of the U. S. Surgeon General National Institutes of Health/NHGRI Centers for Medicare & Medicaid Services Centers for Disease Control and Prevention Health Resources and Services Administration Food and Drug Administration National Institutes of Health/NLM Food and Drug Administration National Institutes of Health/NHLBI HCA, Inc. Department of Veterans Affairs Agency for Healthcare Research and Quality Food and Drug Administration RAND Corporation Agency for Healthcare Research and Quality United. Health Group; SACGHS Harvard Partners Center for Genetics and Genomics Intermountain Healthcare 3

PHC Newborn Screening (NBS) Subgroup Member List • Co-Chairs: – – • Peter van Dyck Steve Downs Health Resources and Services Administration Indiana University, Regenstrief Institute Members: – – – – – – Beryl Crossley* Emory Fry* Betsy Humphreys* Janet Warrington* Cheryl Austein-Casnoff Coleen Boyle Aaron Carroll Mark Carroll Terry Cullen** Otto Del Cid** John Eichwald Greg Feero Alan Fleischman Irene Forsman Roland Gamache** Scott Grosse Alan Hinman Rod Howell Christopher Kus Michele Lloyd-Puryear Joe Martinec American Clinical Laboratory Association, Quest Department of Defense National Institutes of Health (NIH)/National Library of Medicine Affymetrix Inc. Health Resources and Services Administration Centers for Disease Control and Prevention Indiana University, American Academy of Pediatrics Indian Health Service New York City Public Health Laboratory, Association of Public Health Laboratories Centers for Disease Control and Prevention NIH/National Human Genome Research Institute March of Dimes Health Resources and Services Administration Indiana State Department of Health, Association of State and Territorial Health Officials Centers for Disease Control and Prevention Public Health Informatics Institute NIH/National Institute of Child Health and Human Development New York State Department of Health, Association of Maternal and Child Health Programs Health Resources and Services Administration Consumer *Member of the Personalized Health Care Workgroup **Member of the Population Health and Clinical Care Connections Workgroup or designee 4

PHC NBS Subgroup Member List (cont. ) • Members: – – – – • Minnesota Public Health Laboratory, Association of Public Health Laboratories University of North Carolina at Chapel Hill, American Academy of Pediatrics Public Health Informatics Institute Consumer Maryland Department of Health and Mental Hygiene Centers for Disease Control and Prevention American College of Medical Genetics Senior Advisors / Resources: – – – • Mark Mc. Cann** Cindy Powell David Ross Kathy Stagni Linda Vaughan Bob Vogt Mike Watson Rick Friedman Harry Hannon Melanie Lockhart Marie Mann Craig Mason Clem Mc. Donald Michelle Meigs Carolyn Mullen Ken Pass Brad Therrell Karl White Patina Zarcone Centers for Medicare and Medicaid Services Centers for Disease Control and Prevention March of Dimes Health Resources and Services Administration University of Maine NIH/National Library of Medicine Association of Public Health Laboratories March of Dimes New York State Department of Health University of Texas Health Science Center at San Antonio Utah State University Association of Public Health Laboratories Gregory Downing Kristin Brinner Alan Zuckerman Lauren Kim Office of the Secretary, HHS Georgetown University, State Alliance for e-Health Bearing. Point Staff: – – **Member of the Population Health and Clinical Care Connections Workgroup or designee 5

Fostering Information Sharing for NBS • Overarching Goals: – Identify, develop, and encourage adoption of appropriate standards by instrument manufacturers, public health laboratories, and EHR vendors, to facilitate interoperable exchange of newborn screening test results (includes genetic, metabolic, and hearing tests) – Ensure timely communication between state public health laboratories and newborn nurseries doing screening and immediate follow-up, and the primary care professionals and specialists who are involved in the diagnosis, treatment, and management of the infants identified – Potential to have substantial public health benefit (coupled with national goal for standard test performance) and local interest 6

PHC NBS Subgroup Activities to Date • PHC staff presentation at September 5, 2007 PHCCC Workgroup Meeting • Four subgroup meetings held October 2007 through January 2008 – Considered the following topics: • Need for standards in areas of: – Test information (LOINC) – Diagnostic codes (SNOMED, Medcin, ICD, etc. ) – Raw data from lab instruments • Tracking of qualitative (testing and subjective observations), quantitative (numeric values that represent analytic values, percentiles, and/or ratios, accompanied by expected ranges) and interpretive reports to patients, clinicians, and registries • Confidentiality, privacy, and security concerns • Research use of screening data • Examination of other registries (hearing screens, immunization) • Linking of test results with clinical decision support tools 7

PHC NBS Subgroup Activities to Date (cont. ) • Prioritized issues for recommendations development • Fielded NBS survey with state lab programs through the National Newborn Screening and Genetics Resource Center (NNSGRC) • Presented draft recommendations at January 30 PHC Workgroup meeting for consideration – Currently, finalizing draft recommendations and developing a NBS high-level use case 8

Overview of Newborn Screening • Public Health Sector – Blood spot obtained and hearing screening completed – Sample and clinical data sent to NBS laboratory – Testing completed and reports sent to • Primary care provider • Family (sometimes) • Relevant subspecialist (sometimes) • Clinical Sector – Confirmatory testing – Treatment of affected children – Long term follow-up • Regional and National Efforts – NBS system quality improvement – Research on diagnostic testing and treatment 9

Draft Recommendations: NBS Use Case • Recommendation 1. 0: The information flows for Newborn Screening should be prioritized for Use Case Development. All of the multidirectional information flows, stakeholders, and other participants involved in the complete evaluation of newborn screening (i. e. , hearing detection, dried blood spot screening, and diagnostic confirmation) should be considered so that appropriate standards and interoperability specifications can be developed to support information exchange. 10

Draft Recommendations: NBS Use Case (cont. ) – Recommendation 1. 0. 1: The Newborn Screening Subgroup of the Personalized Health Care Workgroup should complete development of a reference matrix of tests, analytes, conditions screened for, and associated genomic variants that are used in newborn screening programs. – Recommendation 1. 0. 2: Based on the reference matrix described in Recommendation 1. 0. 1, appropriate codes should be identified for use in electronic reports to identify the test ordered, individual test results, and categorical results of these tests (e. g. , Logical Observation Identifiers Names and Codes (LOINC), Systematized Nomenclature of Medicine – Clinical Terms (SNOMED-CT), Health Level Seven (HL 7), Online Mendelian in Man (OMIM), International Classification of Diseases – Ninth Edition (ICD-9), and ICD – 10 Clinical Modification (CM)). 11

Draft Recommendations: NBS Use Case (cont. ) – Recommendation 1. 0. 3: Long-term maintenance of the reference matrix should be coordinated by the National Library of Medicine (NLM) in collaboration with the Health Resources and Services Administration (HRSA) and the Centers for Disease Control and Prevention (CDC). – Recommendation 1. 0. 4: For the Use Case development process, ONC should consider the need for documentation of informed consent that authorizes transmittal of results, the need for ongoing collection of information for long-term follow-up, and integration of existing educational and clinical decision support information. 12

Draft Recommendations: NBS Use Case (cont. ) • Recommendation 1. 1: Requirements for electronic reporting of newborn screening results should include specifications for reporting the quantitative measurements that now underpin the qualitative results and/or interpretations. Allowance should be made for accompanying qualitative and/or interpretive reports, and other test- or method-specific information that may assist in qualitative result interpretation. Quantitative reports may include numeric values that represent analytic values, percentiles, and/or ratios and should be accompanied by expected ranges. Qualitative reports may include testing observations (e. g. , fluorescence or no fluorescence) and subjective evaluations (e. g. , Hb FA present). Interpretive reports may include probability information (e. g. , probable Hb S, S anemia), or other reporting information (e. g. , T 4 out-of-range, TSH out-of-range, please refer for serum testing). 13

Draft Recommendations: NBS Use Case (cont. ) – Recommendation 1. 1. 1: HHS should work with the National Governors Association (NGA) and the National Conference of State Legislatures (NCSL) to support electronic reporting of quantitative, qualitative, and/or interpretive reports. 14

Draft Recommendations: NBS Use Case (cont. ) – Recommendation 1. 1. 2: HHS should convene a workgroup with participation from the Centers for Medicare and Medicaid Services (CMS), HRSA, Substance Abuse and Mental Health Services Administration (SAMHSA), Administration for Children and Families (ACF), and other agencies that provide grants or reimbursement to health care providers, in order to determine the most appropriate ways to facilitate the adoption and development of electronic systems that conform to the concepts and standards identified in the Use Case. Special attention should be given to funding opportunities provided by existing authorities associated with the Early, Periodic, Screening, Diagnostic and Testing (EPSDT) requirements under Title XIX for Medicaid beneficiaries; e. g. , enhanced match for the Medicaid Management Information System (MMIS) and in a manner consistent with the emerging architectures described within the Medicaid Information Technology Architecture (MITA). 15

Draft Recommendations: NBS Use Case (cont. ) • Recommendation 1. 2: An action plan, timetable, and metrics for the implementation and tracking of these recommendations should be developed by HRSA to measure uptake of electronic transmission of test results that conform to the standards identified through the Use Case development process. HRSA Newborn Screening technical support centers should conduct annual surveys to monitor the pace of implementing these recommendations, standards, and transmission of newborn tests results by electronic means (EHRs and repositories). 16

Draft Recommendations: Confidentiality, Privacy, and Security Issues Specific to NBS • Recommendation 2. 0: HHS should work with state stakeholders to accurately identify, analyze, and develop solutions to address any misperceptions or misapplications of state privacy laws that may affect the timely transmission of newborn screening results. This work should also include an analysis of whether clarifying guidance from HHS related to the Health Insurance Portability and Accountability Act (HIPAA) Privacy and Security Rules, the Clinical Laboratory Improvement Amendments (CLIA), and other regulations under HHS’ authority would be appropriate. 17

Draft Recommendations: Reporting of NBS Results to Improve Population Health • Recommendation 3. 0: A taskforce that includes representatives from appropriate federal and state agencies, professional and public organizations, and the Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children (ACHDGDNC) should be formed to develop a plan for and descriptions of a patient-based information registry of newborn screening data within twelve months. Public review of the findings of this taskforce will be essential to affirm the ethical nature of any proposed research that will be facilitated by the development of electronic test reporting and national standards for identifying the tests performed and results obtained. 18

NBS Draft High-Level Use Case • This use case is organized primarily around four scenarios: – – • The four scenarios address the following perspectives: – – – • Screening Test Performance Diagnostic Confirmation and Short Term Follow-up Clinical/Social Management Population Health Management and Long Term Follow-up Birthing Facility Testing Laboratories Medical Home/Primary Care Provider Diagnostic Center Public Health This document will be used to support any formal use case development through the Office of the National Coordinator for Health IT (ONC) – There would be multiple opportunities for public comment 19

Next Steps • • • February 6: NBS Subgroup meeting to provide additional input on NBS draft recommendations February 6: PHC staff presentation to PHCCC Workgroup for comments February 14: PHC Workgroup meeting to finalize recommendations February 26: Once consensus is reached, PHC Workgroup Co-chairs to advance recommendations to the AHIC Ongoing: Subgroup to continue work on the following – High-level use case for NBS • Describes the information flow for both newborn dried blood spot screening and early hearing detection and intervention – NBS matrix for standards development processes • Reference matrix of tests, analytes, conditions screened for, and associated genomic variants that are used in newborn screening programs 20
 Jenis sistem informasi
Jenis sistem informasi Thorsten butz
Thorsten butz Workgroup sphere of influence
Workgroup sphere of influence Workgroup sphere of influence
Workgroup sphere of influence Diff between workgroup and domain
Diff between workgroup and domain Workgroup database adalah bentuk database
Workgroup database adalah bentuk database Shoretel salesforce
Shoretel salesforce Off the shelf application software examples
Off the shelf application software examples Vrs elite workgroup
Vrs elite workgroup Advantages and disadvantages of speed networking
Advantages and disadvantages of speed networking Workgroup vs domain pros and cons
Workgroup vs domain pros and cons Personal application software
Personal application software Poverty reduction workgroup
Poverty reduction workgroup Perbedaan workgroup dan enterprise
Perbedaan workgroup dan enterprise Ladder safety training ppt
Ladder safety training ppt Workgroup cluster
Workgroup cluster Primary secondary tertiary care nursing
Primary secondary tertiary care nursing Hexiangnan
Hexiangnan Personalised mobile search engine
Personalised mobile search engine Pentaho personalized demo
Pentaho personalized demo Personalized patient education
Personalized patient education