ADVANCE Main Results Secondary Outcomes Allcause mortality Cumulative














- Slides: 42

ADVANCE Main Results Secondary Outcomes

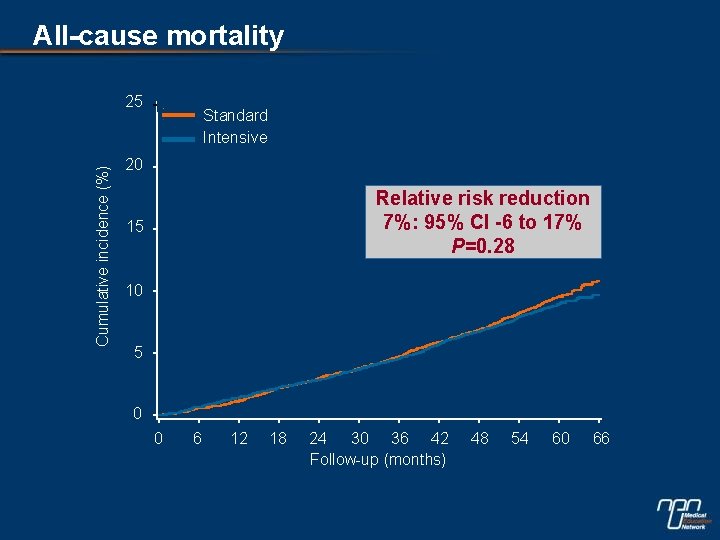
All-cause mortality Cumulative incidence (%) 25 Standard Intensive 20 Relative risk reduction 7%: 95% CI -6 to 17% P=0. 28 15 10 5 0 0 6 12 18 24 30 36 42 Follow-up (months) 48 54 60 66

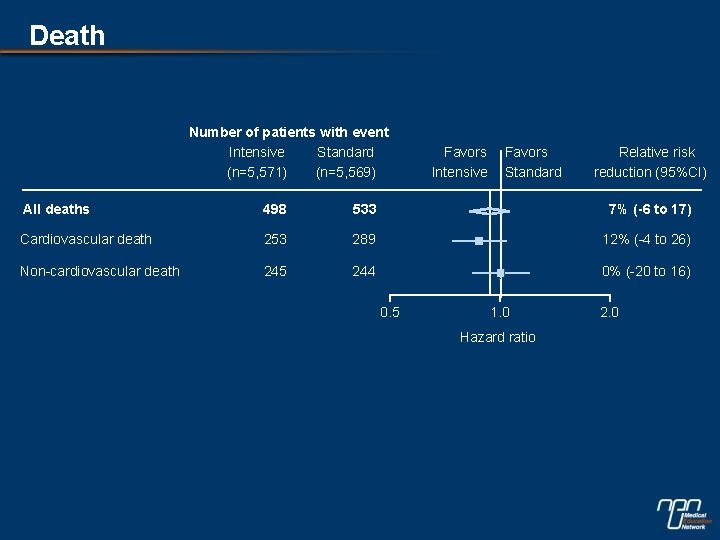
Death Number of patients with event Intensive Standard (n=5, 571) (n=5, 569) Favors Intensive Favors Standard Relative risk reduction (95%CI) All deaths 498 533 7% (-6 to 17) Cardiovascular death 253 289 12% (-4 to 26) Non-cardiovascular death 245 244 0% (-20 to 16) 0. 5 1. 0 Hazard ratio 2. 0

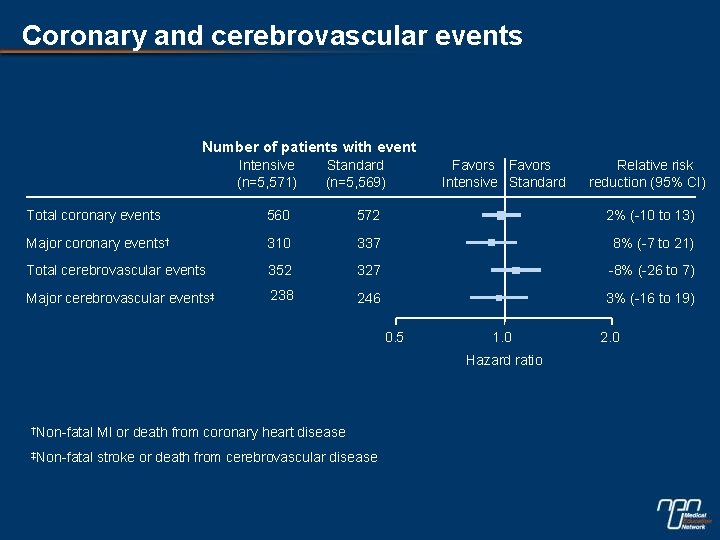
Coronary and cerebrovascular events Number of patients with event Intensive (n=5, 571) Standard (n=5, 569) Favors Intensive Standard Relative risk reduction (95% CI) Total coronary events 560 572 2% (-10 to 13) Major coronary events† 310 337 8% (-7 to 21) Total cerebrovascular events 352 327 -8% (-26 to 7) Major cerebrovascular events‡ 238 246 3% (-16 to 19) 0. 5 1. 0 Hazard ratio †Non-fatal MI or death from coronary heart disease ‡Non-fatal stroke or death from cerebrovascular disease 2. 0

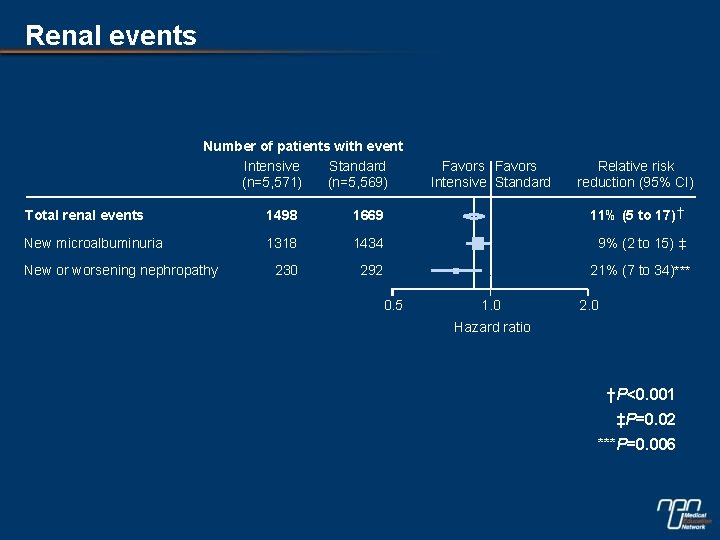
Renal events Number of patients with event Intensive Standard (n=5, 571) (n=5, 569) Favors Intensive Standard Relative risk reduction (95% CI) Total renal events 1498 1669 11% (5 to 17)† New microalbuminuria 1318 1434 9% (2 to 15) ‡ 230 292 21% (7 to 34)*** New or worsening nephropathy 0. 5 1. 0 2. 0 Hazard ratio †P<0. 001 ‡P=0. 02 ***P=0. 006

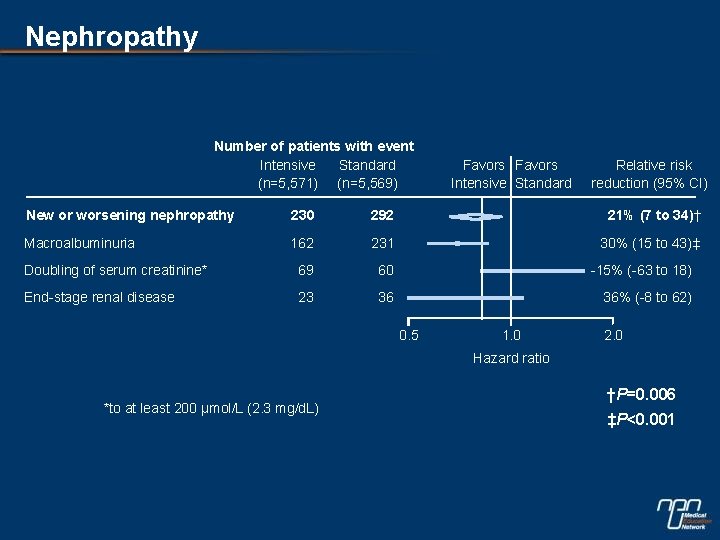
Nephropathy Number of patients with event Intensive Standard (n=5, 571) (n=5, 569) Favors Intensive Standard Relative risk reduction (95% CI) New or worsening nephropathy 230 292 21% (7 to 34)† Macroalbuminuria 162 231 30% (15 to 43)‡ Doubling of serum creatinine* 69 60 -15% (-63 to 18) End-stage renal disease 23 36 36% (-8 to 62) 0. 5 1. 0 2. 0 Hazard ratio *to at least 200 μmol/L (2. 3 mg/d. L) †P=0. 006 ‡P<0. 001

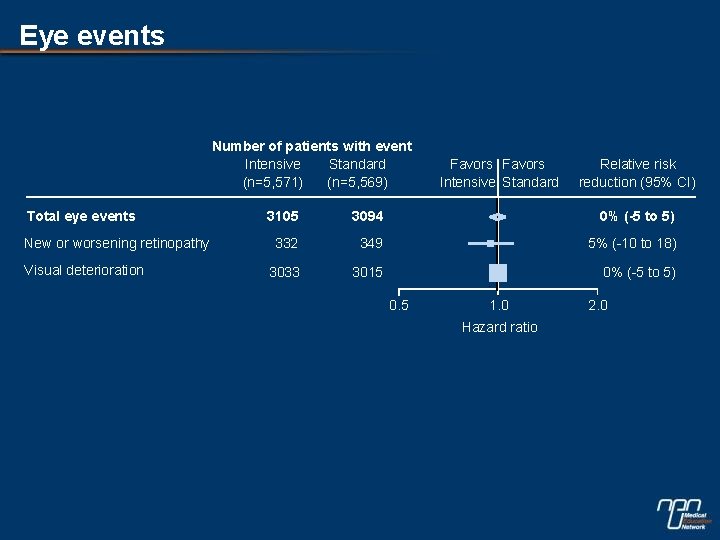
Eye events Number of patients with event Intensive Standard (n=5, 571) (n=5, 569) Total eye events New or worsening retinopathy Visual deterioration Favors Intensive Standard Relative risk reduction (95% CI) 3105 3094 0% (-5 to 5) 332 349 5% (-10 to 18) 3033 3015 0% (-5 to 5) 0. 5 1. 0 Hazard ratio 2. 0

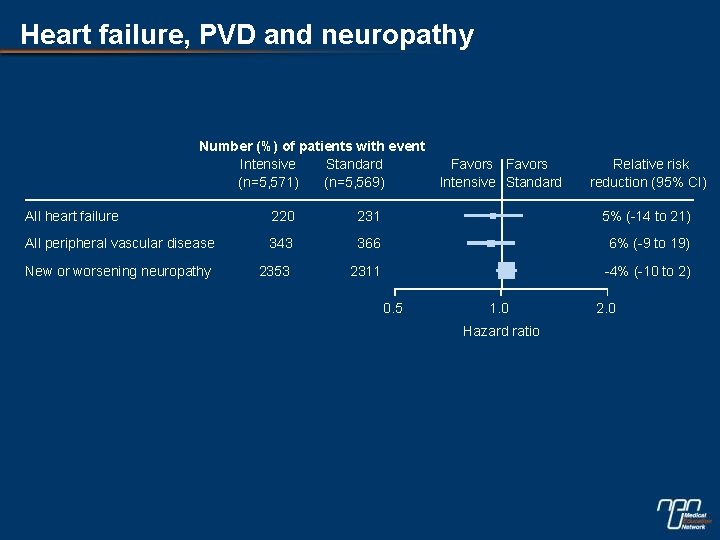
Heart failure, PVD and neuropathy Number (%) of patients with event Intensive Standard (n=5, 571) (n=5, 569) Favors Intensive Standard Relative risk reduction (95% CI) All heart failure 220 231 5% (-14 to 21) All peripheral vascular disease 343 366 6% (-9 to 19) New or worsening neuropathy 2353 2311 -4% (-10 to 2) 0. 5 1. 0 Hazard ratio 2. 0

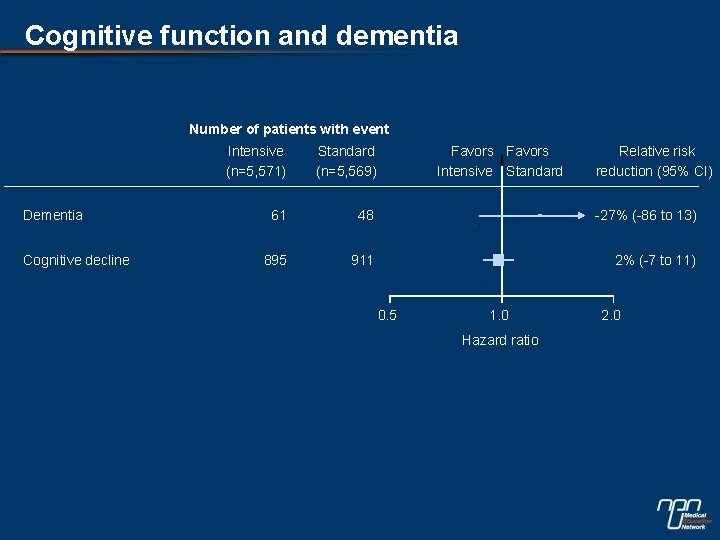
Cognitive function and dementia Number of patients with event Dementia Cognitive decline Intensive (n=5, 571) Standard (n=5, 569) Favors Intensive Standard 61 48 -27% (-86 to 13) 895 911 2% (-7 to 11) 0. 5 1. 0 Hazard ratio Relative risk reduction (95% CI) 2. 0

ADVANCE Effects in Patient Subgroups

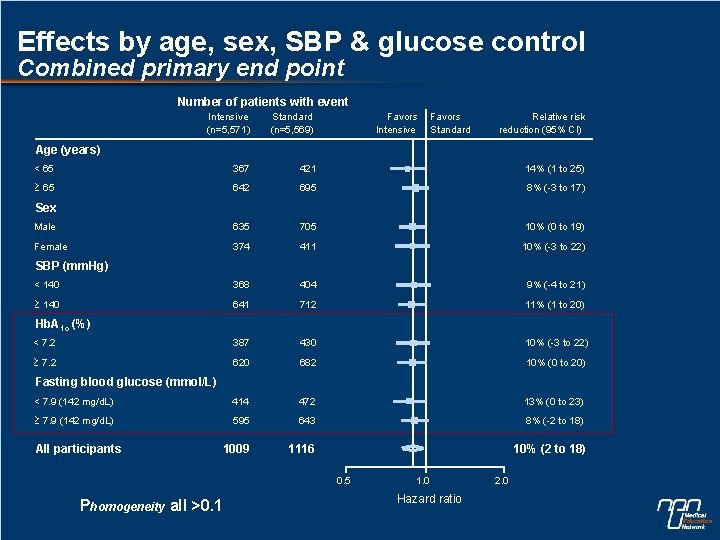
Effects by age, sex, SBP & glucose control Combined primary end point Number of patients with event Intensive (n=5, 571) Standard (n=5, 569) Favors Intensive Favors Standard Relative risk reduction (95% CI) < 65 367 421 14% (1 to 25) 65 642 695 8% (-3 to 17) Male 635 705 10% (0 to 19) Female 374 411 10% (-3 to 22) < 140 368 404 9% (-4 to 21) 140 641 712 11% (1 to 20) < 7. 2 387 430 10% (-3 to 22) 7. 2 620 682 10% (0 to 20) < 7. 9 (142 mg/d. L) 414 472 13% (0 to 23) 7. 9 (142 mg/d. L) 595 643 8% (-2 to 18) 1009 1116 10% (2 to 18) Age (years) Sex SBP (mm. Hg) Hb. A 1 c (%) Fasting blood glucose (mmol/L) All participants 0. 5 Phomogeneity all >0. 1 1. 0 Hazard ratio 2. 0

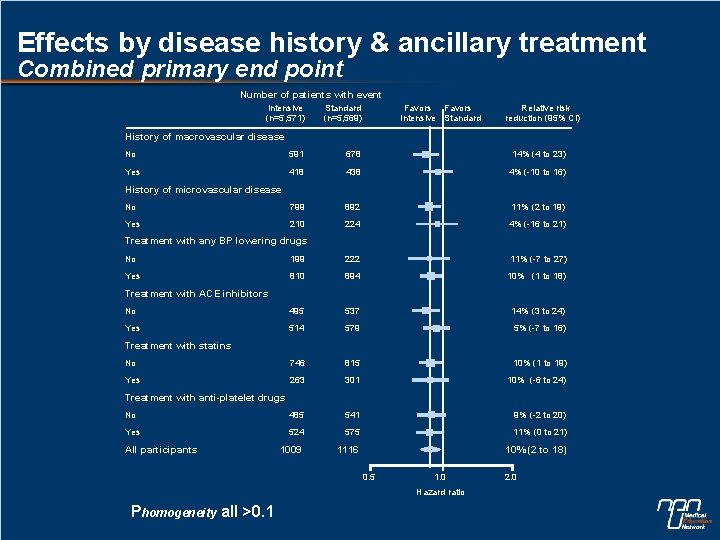
Effects by disease history & ancillary treatment Combined primary end point Number of patients with event Intensive (n=5, 571) Standard (n=5, 569) Favors Intensive Standard Relative risk reduction (95% CI) History of macrovascular disease No 591 678 14% (4 to 23) Yes 418 438 4% (-10 to 16) No 799 892 11% (2 to 19) Yes 210 224 4% (-16 to 21) History of microvascular disease Treatment with any BP lowering drugs No 199 222 11% (-7 to 27) Yes 810 894 10% (1 to 18) No 495 537 14% (3 to 24) Yes 514 579 5% (-7 to 16) No 746 815 10% (1 to 19) Yes 263 301 10% (-6 to 24) No 485 541 9% (-2 to 20) Yes 524 575 11% (0 to 21) 1009 1116 10% (2 to 18) Treatment with ACE inhibitors Treatment with statins Treatment with anti-platelet drugs All participants 0. 5 1. 0 Hazard ratio Phomogeneity all >0. 1 2. 0

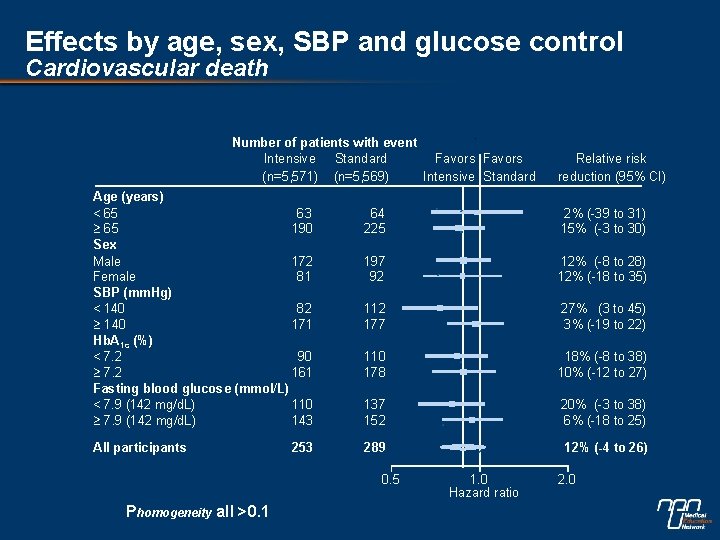
Effects by age, sex, SBP and glucose control Cardiovascular death Number of patients with event Intensive Standard Favors (n=5, 571) (n=5, 569) Intensive Standard Age (years) < 65 63 65 190 Sex Male 172 Female 81 SBP (mm. Hg) < 140 82 140 171 Hb. A 1 c (%) < 7. 2 90 7. 2 161 Fasting blood glucose (mmol/L) < 7. 9 (142 mg/d. L) 110 7. 9 (142 mg/d. L) 143 All participants 253 64 225 2% (-39 to 31) 15% (-3 to 30) 197 92 12% (-8 to 28) 12% (-18 to 35) 112 177 27% (3 to 45) 3% (-19 to 22) 110 178 18% (-8 to 38) 10% (-12 to 27) 137 152 20% (-3 to 38) 6% (-18 to 25) 289 12% (-4 to 26) 0. 5 Phomogeneity all >0. 1 Relative risk reduction (95% CI) 1. 0 Hazard ratio 2. 0

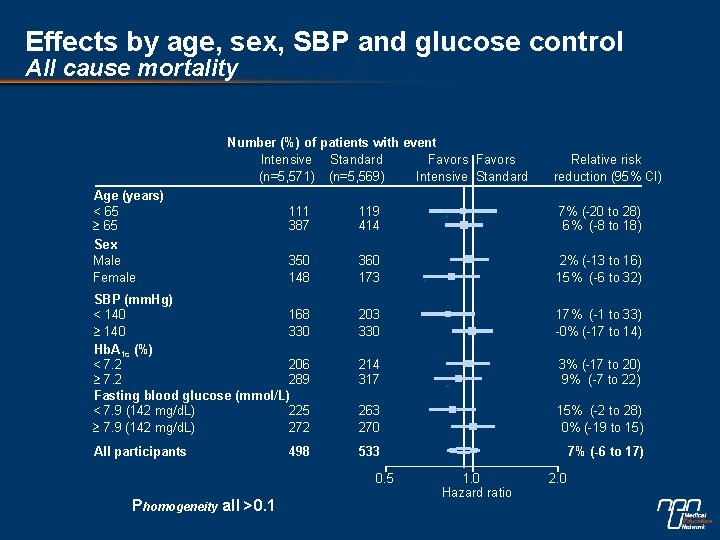
Effects by age, sex, SBP and glucose control All cause mortality Number (%) of patients with event Intensive Standard Favors (n=5, 571) (n=5, 569) Intensive Standard Age (years) < 65 Sex Male Female 111 387 119 414 7% (-20 to 28) 6% (-8 to 18) 350 148 360 173 2% (-13 to 16) 15% (-6 to 32) 203 330 17% (-1 to 33) -0% (-17 to 14) 214 317 3% (-17 to 20) 9% (-7 to 22) 263 270 15% (-2 to 28) 0% (-19 to 15) 533 7% (-6 to 17) SBP (mm. Hg) < 140 168 140 330 Hb. A 1 c (%) < 7. 2 206 7. 2 289 Fasting blood glucose (mmol/L) < 7. 9 (142 mg/d. L) 225 7. 9 (142 mg/d. L) 272 All participants 498 0. 5 Phomogeneity all >0. 1 Relative risk reduction (95% CI) 1. 0 Hazard ratio 2. 0

Subgroup effects n No evidence of different effects in patient subgroups for the combined primary outcome, cardiovascular death or total mortality

ADVANCE Main Results Other risk factors and concomitant treatments

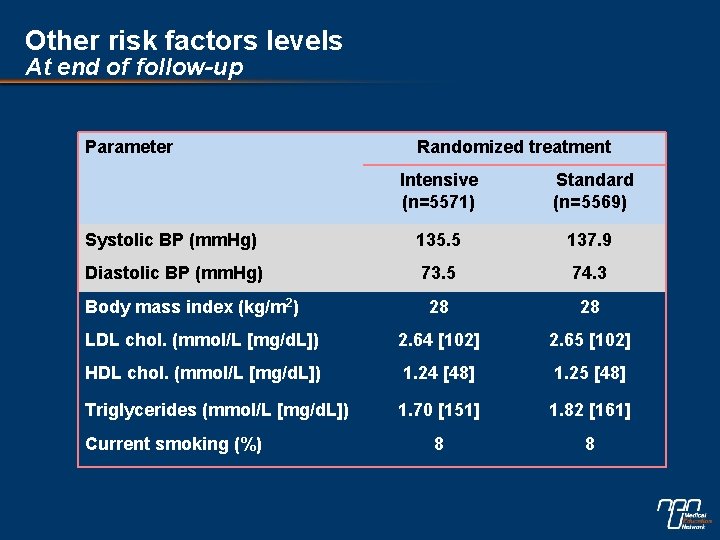
Other risk factors levels At end of follow-up Parameter Randomized treatment Intensive (n=5571) Standard (n=5569) Systolic BP (mm. Hg) 135. 5 137. 9 Diastolic BP (mm. Hg) 73. 5 74. 3 28 28 LDL chol. (mmol/L [mg/d. L]) 2. 64 [102] 2. 65 [102] HDL chol. (mmol/L [mg/d. L]) 1. 24 [48] 1. 25 [48] Triglycerides (mmol/L [mg/d. L]) 1. 70 [151] 1. 82 [161] 8 8 Body mass index (kg/m 2) Current smoking (%)

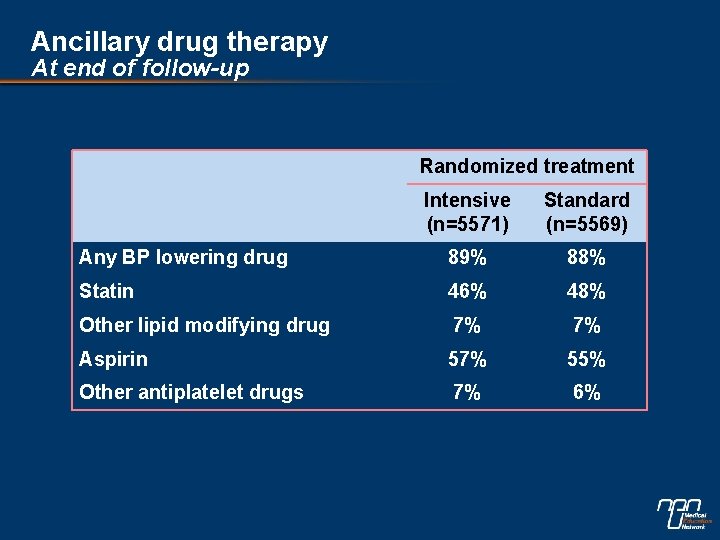
Ancillary drug therapy At end of follow-up Randomized treatment Intensive (n=5571) Standard (n=5569) Any BP lowering drug 89% 88% Statin 46% 48% Other lipid modifying drug 7% 7% Aspirin 57% 55% Other antiplatelet drugs 7% 6%

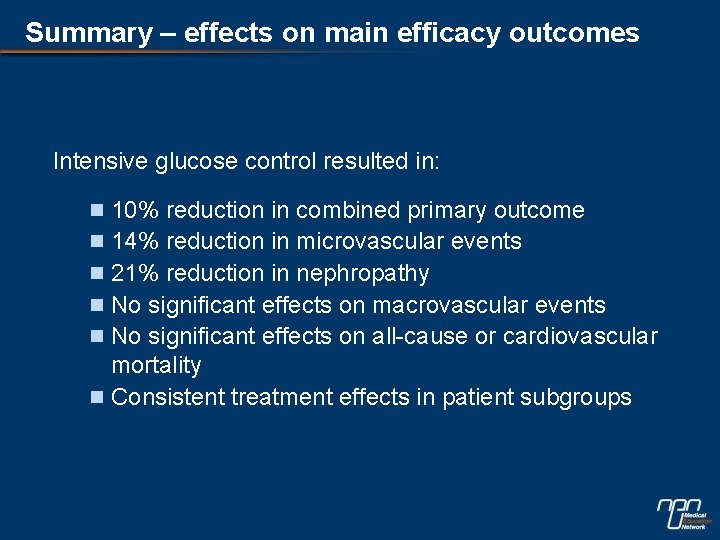
Summary – effects on main efficacy outcomes Intensive glucose control resulted in: n 10% reduction in combined primary outcome n 14% reduction in microvascular events n 21% reduction in nephropathy n No significant effects on macrovascular events n No significant effects on all-cause or cardiovascular mortality n Consistent treatment effects in patient subgroups

ADVANCE Hypoglycemia

Definition § A blood glucose level <2. 8 mmol/L (50 mg/d. L) and/or an episode with typical symptoms and signs of hypoglycemia, without other apparent cause § SEVERE where an individual had transient central nervous system dysfunction and was unable to treat him/herself (required help from another person) § MINOR where the individual was able to treat him/herself

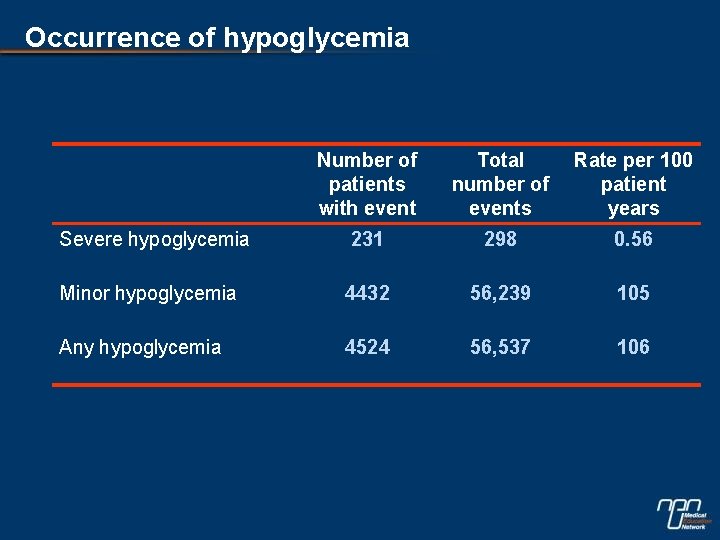
Occurrence of hypoglycemia Number of patients with event Total number of events Rate per 100 patient years Severe hypoglycemia 231 298 0. 56 Minor hypoglycemia 4432 56, 239 105 Any hypoglycemia 4524 56, 537 106

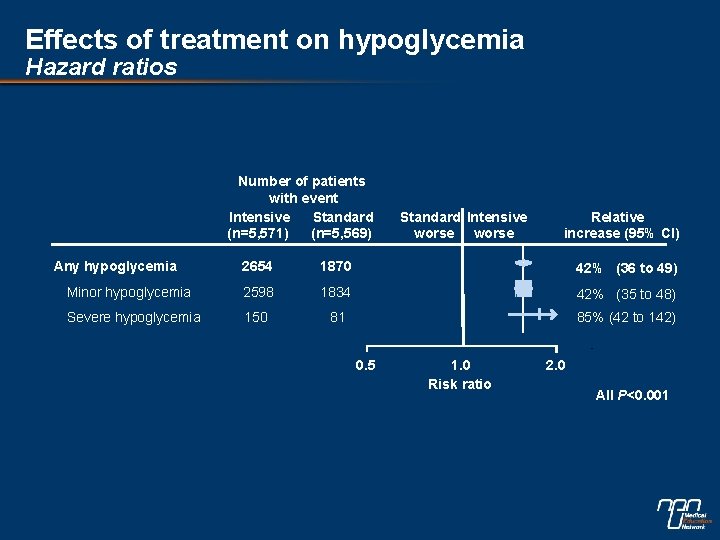
Effects of treatment on hypoglycemia Hazard ratios Number of patients with event Intensive Standard (n=5, 571) (n=5, 569) Any hypoglycemia Standard Intensive worse Relative increase (95% CI) 2654 1870 42% (36 to 49) Minor hypoglycemia 2598 1834 42% (35 to 48) Severe hypoglycemia 150 81 85% (42 to 142) 0. 5 1. 0 Risk ratio 2. 0 All P<0. 001

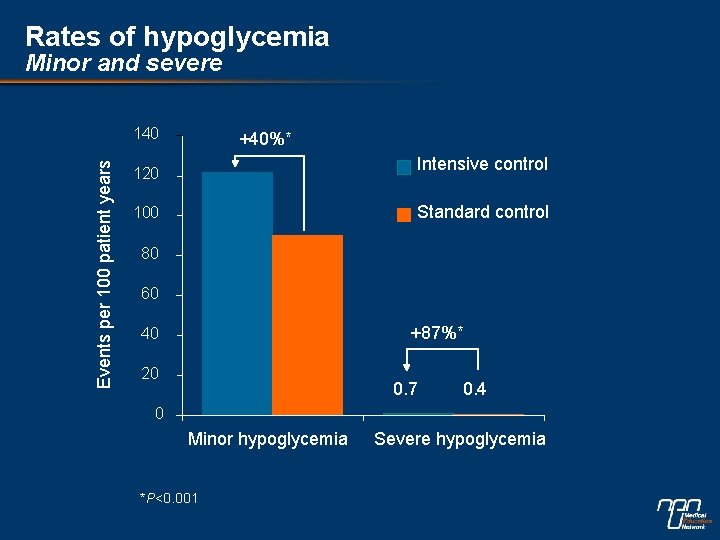
Rates of hypoglycemia Minor and severe Events per 100 patient years 140 +40%* Intensive control 120 Standard control 100 80 60 +87%* 40 20 0. 7 0. 4 0 Minor hypoglycemia *P<0. 001 Severe hypoglycemia

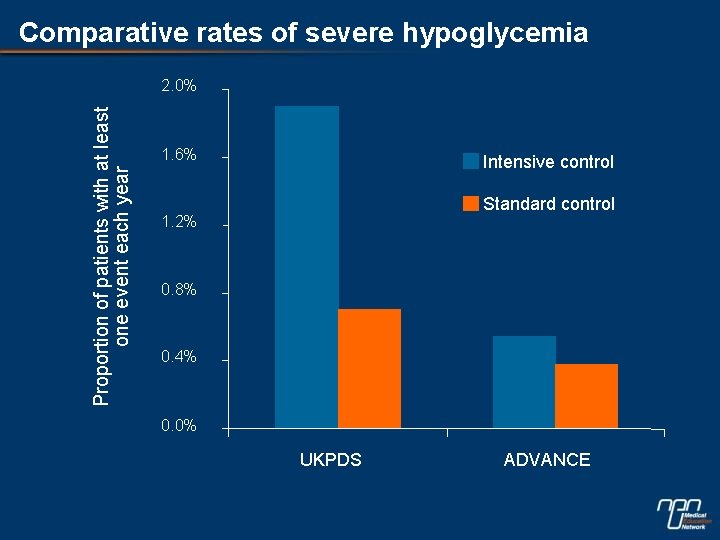
Comparative rates of severe hypoglycemia Proportion of patients with at least one event each year 2. 0% 1. 6% Intensive control Standard control 1. 2% 0. 8% 0. 4% 0. 0% UKPDS ADVANCE

Fatal or disabling sequelae of severe hypoglycemia § One fatal episode in the standard control group § One episode leading to permanent disability in each of the standard and intensive groups

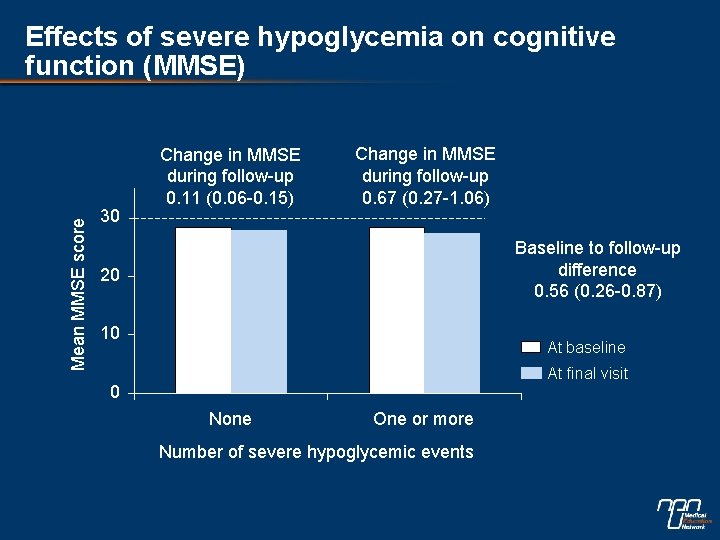
Mean MMSE score Effects of severe hypoglycemia on cognitive function (MMSE) 30 Change in MMSE during follow-up 0. 11 (0. 06 -0. 15) Change in MMSE during follow-up 0. 67 (0. 27 -1. 06) Baseline to follow-up difference 0. 56 (0. 26 -0. 87) 20 10 At baseline At final visit 0 None One or more Number of severe hypoglycemic events

Summary - hypoglycemia § Intensive glucose control increased the risk of severe hypoglycemia § Rates of severe hypoglycemia were low § No significant long-term sequelae were identified § More than half of patients reported neither minor or severe hypoglycemia

ADVANCE Body Weight

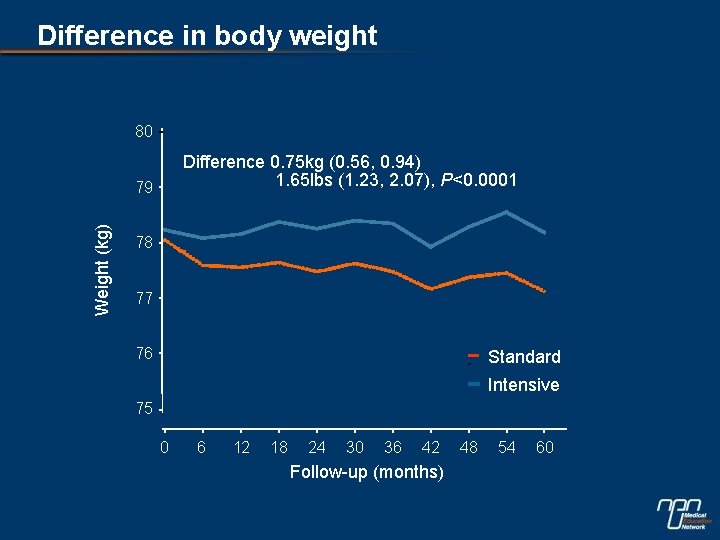
Difference in body weight 80 Difference 0. 75 kg (0. 56, 0. 94) 1. 65 lbs (1. 23, 2. 07), P<0. 0001 Weight (kg) 79 78 77 76 Standard Intensive 75 0 6 12 18 24 30 36 42 Follow-up (months) 48 54 60

Impact of ethnicity Asian § Mean baseline weight = 67 kg (145 lbs) § Mean weight difference = 0. 44 kg (0. 97 lbs) § 0. 7% of baseline weight Non-Asian § Mean baseline weight = 85 kg (187 lbs) § Mean weight difference = 0. 96 kg (2. 1 lbs) § 1. 1% of baseline weight

Impact of follow-up duration UKPDS § Weight gain about 3. 1 kg (6. 8 lbs) § Mostly in first few years after diagnosis § Follow-up 10 years ADVANCE § No weight gain § Weight difference 0. 75 kg (1. 65 lbs) § Follow-up 5 years § Weight difference projected to 10 years = 1. 8 kg (4. 0 lbs)

Summary - weight § No weight gain in ADVANCE § Weight difference between randomized groups less in ADVANCE than UKPDS § Difference likely explained in part at least by ethnicity, stage of disease and duration of follow-up

Balance of risk and benefits Five years treatment § Prevents one vascular event among every 52 patients (mostly renal events) § Causes one severe hypoglycemic event among every 81 patients

ADVANCE in the context of other studies

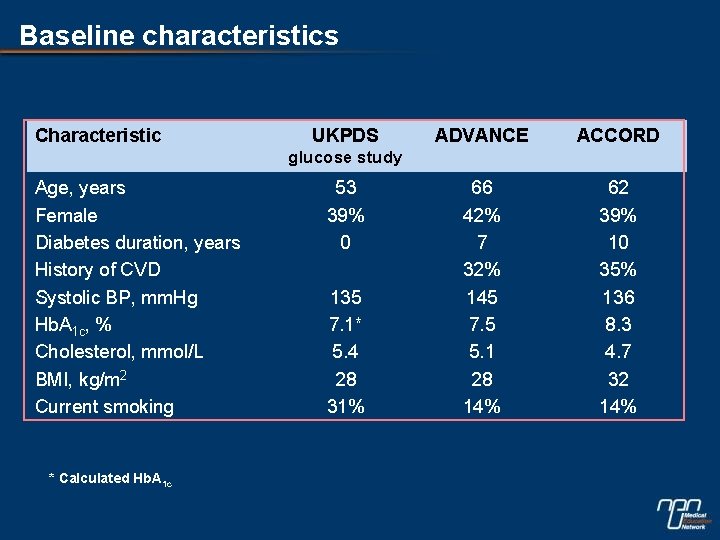
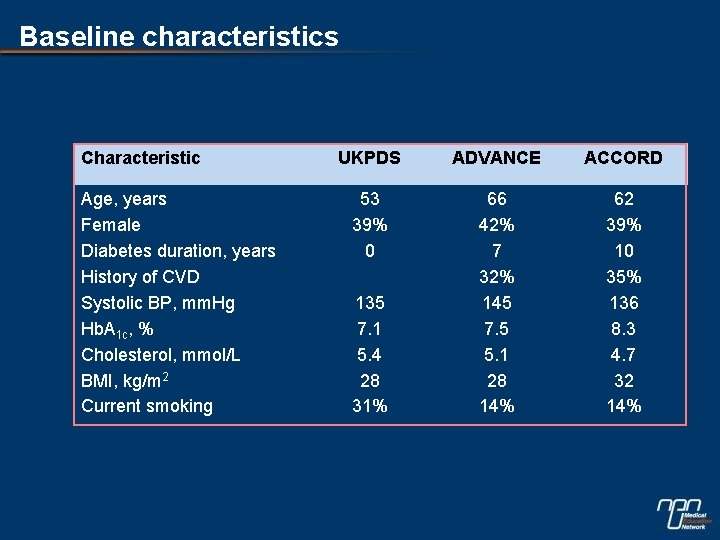
Baseline characteristics Characteristic UKPDS ADVANCE ACCORD 66 42% 7 32% 145 7. 5 5. 1 28 14% 62 39% 10 35% 136 8. 3 4. 7 32 14% glucose study Age, years Female Diabetes duration, years History of CVD Systolic BP, mm. Hg Hb. A 1 c, % Cholesterol, mmol/L BMI, kg/m 2 Current smoking * Calculated Hb. A 1 c 53 39% 0 135 7. 1* 5. 4 28 31%

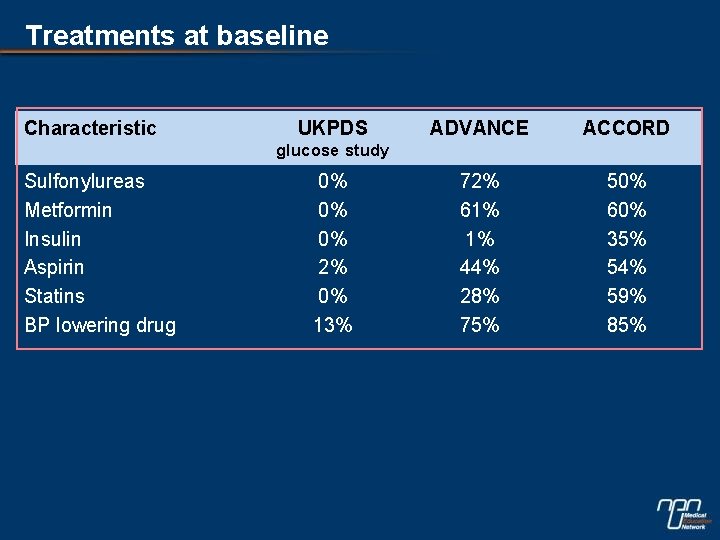
Treatments at baseline Characteristic UKPDS ADVANCE ACCORD 72% 61% 1% 44% 28% 75% 50% 60% 35% 54% 59% 85% glucose study Sulfonylureas Metformin Insulin Aspirin Statins BP lowering drug 0% 0% 0% 2% 0% 13%

ACCORD UKPDS

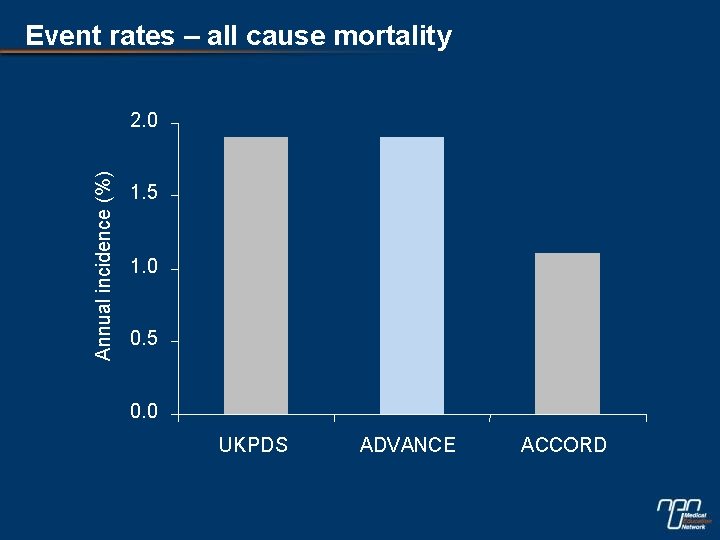
Event rates – all cause mortality Annual incidence (%) 2. 0 1. 5 1. 0 0. 5 0. 0 UKPDS ADVANCE ACCORD

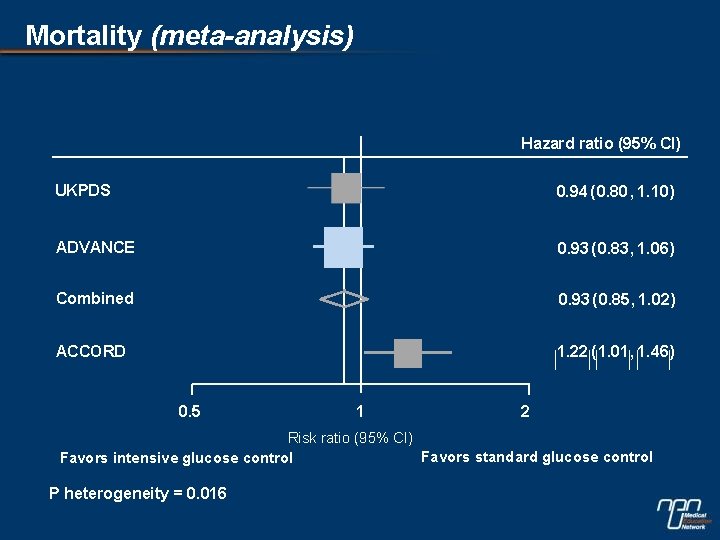
Mortality (meta-analysis) Hazard ratio (95% CI) UKPDS 0. 94 (0. 80 , 1. 10) ADVANCE 0. 93 ( 0. 83, 1. 06) Combined 0. 93 ( 0. 85, 1. 02) ACCORD 1. 22 ( 1. 01, 1. 46) 0. 5 1 2 Risk ratio (95% CI) Favors standard glucose control Favors intensive glucose control P heterogeneity = 0. 016

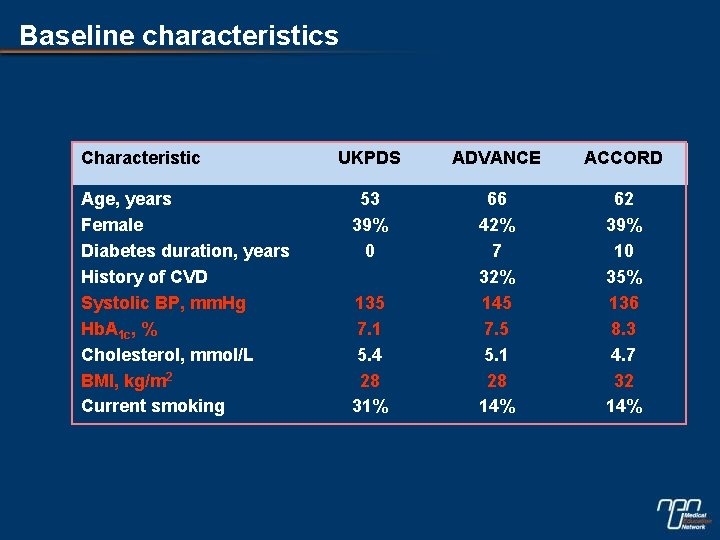
Baseline characteristics Characteristic Age, years Female Diabetes duration, years History of CVD Systolic BP, mm. Hg Hb. A 1 c, % Cholesterol, mmol/L BMI, kg/m 2 Current smoking UKPDS ADVANCE ACCORD 53 39% 0 66 42% 7 32% 145 7. 5 5. 1 28 14% 62 39% 10 35% 136 8. 3 4. 7 32 14% 135 7. 1 5. 4 28 31%

Baseline characteristics Characteristic Age, years Female Diabetes duration, years History of CVD Systolic BP, mm. Hg Hb. A 1 c, % Cholesterol, mmol/L BMI, kg/m 2 Current smoking UKPDS ADVANCE ACCORD 53 39% 0 66 42% 7 32% 145 7. 5 5. 1 28 14% 62 39% 10 35% 136 8. 3 4. 7 32 14% 135 7. 1 5. 4 28 31%
 Flacker score
Flacker score What is the central idea of the great gatsby
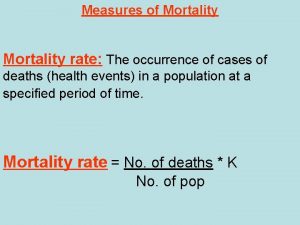
What is the central idea of the great gatsby Disease specific mortality rate formula
Disease specific mortality rate formula Imr equation
Imr equation Perinatal mortality rate
Perinatal mortality rate Proportionate mortality rate formula
Proportionate mortality rate formula Age adjusted mortality rate
Age adjusted mortality rate Indirect age adjustment
Indirect age adjustment Age adjusted mortality rate definition
Age adjusted mortality rate definition Attributable mortality
Attributable mortality Calculate relative risk
Calculate relative risk Continuous mortality investigation
Continuous mortality investigation Rumus morbiditas
Rumus morbiditas Infant mortality rate formula
Infant mortality rate formula Morality play genre
Morality play genre Infant mortality rate formula
Infant mortality rate formula Menghitung angka kelahiran kasar
Menghitung angka kelahiran kasar The cause-specific mortality rate from roller-skating was:
The cause-specific mortality rate from roller-skating was: Neonatal mortality rate formula
Neonatal mortality rate formula Morbidity and mortality difference
Morbidity and mortality difference Risk behaviours definition pdhpe
Risk behaviours definition pdhpe Nir ap human geography
Nir ap human geography Form of will future
Form of will future Example of main idea
Example of main idea Void main int main
Void main int main Proxytotal
Proxytotal In advance of the broken arm
In advance of the broken arm катетер export advance
катетер export advance Advance science
Advance science What are the types of advance directives
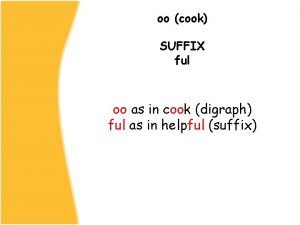
What are the types of advance directives Suffix of cook
Suffix of cook Enduring understanding definition
Enduring understanding definition Nature advance online publication
Nature advance online publication Skimming advance organizer examples
Skimming advance organizer examples Lexis advance reviews
Lexis advance reviews Difference between loan and advance
Difference between loan and advance Planning is deciding in advance what is to be done
Planning is deciding in advance what is to be done Skimming advance graphic organizer
Skimming advance graphic organizer Skimming organizer example
Skimming organizer example Advance case management
Advance case management Advance pricing agreement
Advance pricing agreement Advance organizer
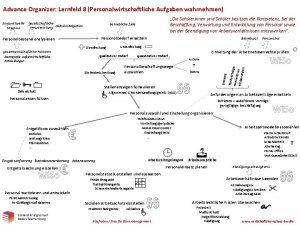
Advance organizer Personalwirtschaftliche aufgaben wahrnehmen
Personalwirtschaftliche aufgaben wahrnehmen