A Randomized Controlled Trial of Male Circumcision to













- Slides: 19

A Randomized Controlled Trial of Male Circumcision to Reduce HIV Incidence in Kisumu, Kenya Robert C. Bailey Univ. of Illinois at Chicago J. O. Ndinya-Achola Univ. of Nairobi Stephen Moses University of Manitoba Kawango Agot UNIM Project/ IMPACT Ian Maclean University of Manitoba John Krieger University of Washington Corette Parker RTI International Supported by Division of AIDS, NIAID, NIH and the Canadian Institute for Health Research

Hypotheses · Circumcision will reduce HIV incidence among men aged 18 -24 years by 50%. · Circumcision will result in less than a 2% rate of significant post-surgical complications requiring follow-up care. · There will be no difference between circumcised men and controls in reported sexual behaviour following the circumcision procedure.

Study Population · Approximately 2, 600, 000 Luo live in Nyanza Province, western Kenya. · Luo do not practice traditional circumcision. – 9. 9% of Luo men are circumcised. · Kisumu District has approximately 39, 000 men aged 18 -24 years. · 86% live within 30 minutes of central Kisumu City.

Clients of STD clinics Clients of VCT centres Organizations (boda, jua kali, car washers) Peer recruiters Fliers, radio, shows Beaches, discos, rural communities

Inclusion and Exclusion Criteria Inclusion Criteria Exclusion Criteria · Uncircumcised · Foreskin covers less than half of glans · HIV negative · Sexually active · Aged 18 -24 years · Hemophiliac or other bleeding disorder · Resident of Kisumu District · High Prothrombin Time Index. · No plans to move for at least the next 2 years. · Consent to participate · Other medical condition contra-indicating surgery · Hemoglobin over 9. 0 grams per 100 ml · Absolute indication for circumcision

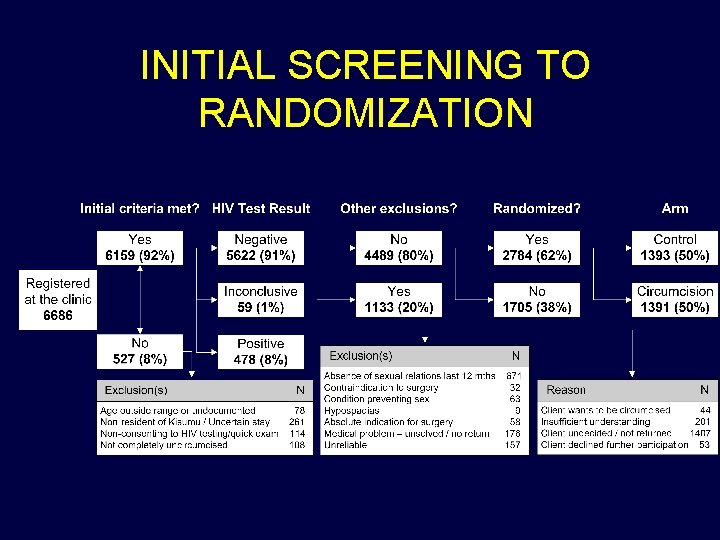
INITIAL SCREENING TO RANDOMIZATION

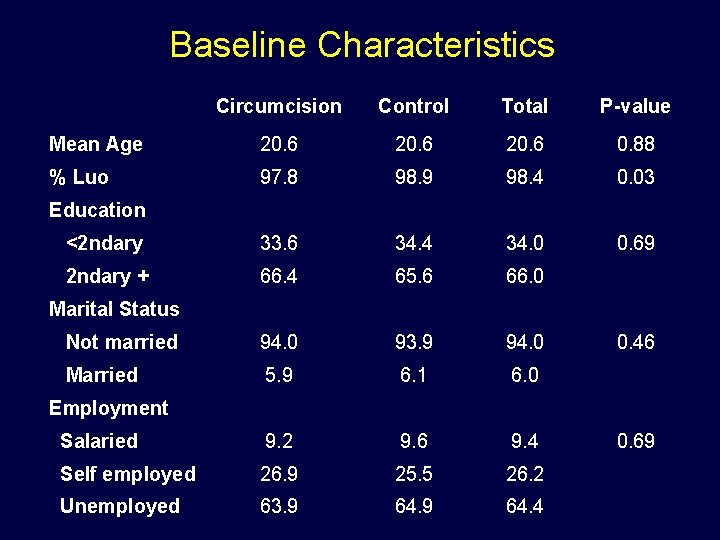
Baseline Characteristics Circumcision Control Total P-value Mean Age 20. 6 0. 88 % Luo 97. 8 98. 9 98. 4 0. 03 <2 ndary 33. 6 34. 4 34. 0 0. 69 2 ndary + 66. 4 65. 6 66. 0 Not married 94. 0 93. 9 94. 0 Married 5. 9 6. 1 6. 0 Salaried 9. 2 9. 6 9. 4 Self employed 26. 9 25. 5 26. 2 Unemployed 63. 9 64. 4 Education Marital Status 0. 46 Employment 0. 69

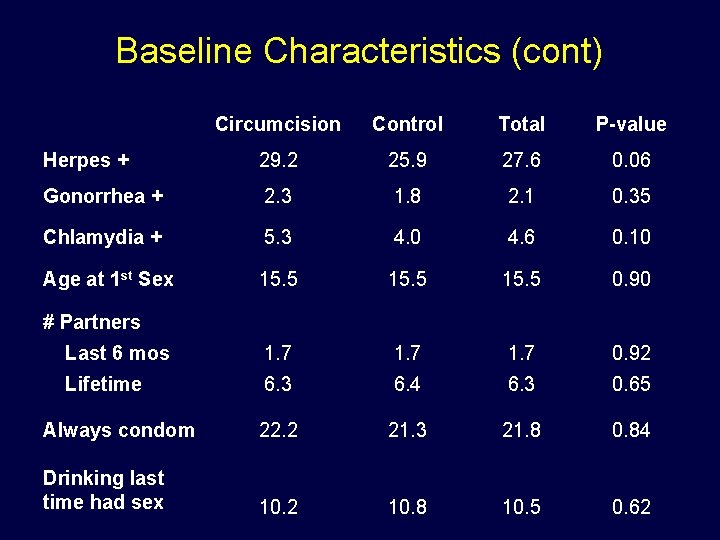
Baseline Characteristics (cont) Circumcision Control Total P-value Herpes + 29. 2 25. 9 27. 6 0. 06 Gonorrhea + 2. 3 1. 8 2. 1 0. 35 Chlamydia + 5. 3 4. 0 4. 6 0. 10 Age at 1 st Sex 15. 5 0. 90 Last 6 mos 1. 7 0. 92 Lifetime 6. 3 6. 4 6. 3 0. 65 Always condom 22. 2 21. 3 21. 8 0. 84 Drinking last time had sex 10. 2 10. 8 10. 5 0. 62 # Partners

HIV Prevalence by Age Percent HIV+ N = 4, 643 Age in Years

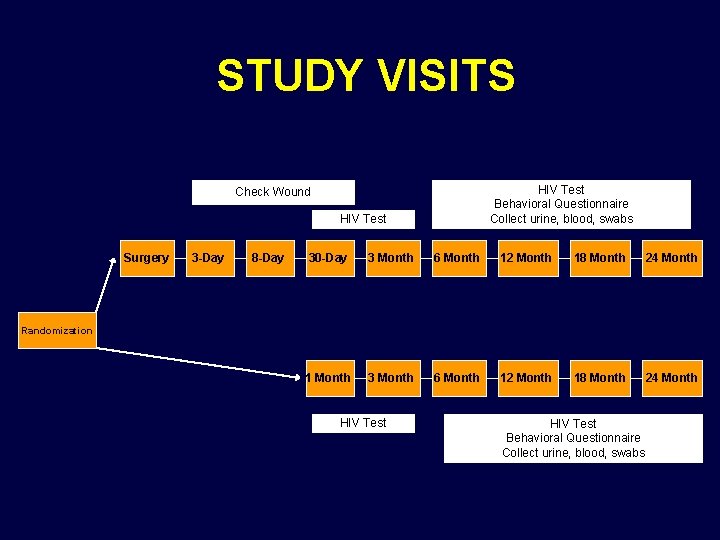
STUDY VISITS HIV Test Behavioral Questionnaire Collect urine, blood, swabs Check Wound HIV Test Surgery 3 -Day 8 -Day 30 -Day 3 Month 6 Month 12 Month 18 Month 24 Month 1 Month 3 Month 6 Month 12 Month 18 Month 24 Month Randomization HIV Test Behavioral Questionnaire Collect urine, blood, swabs

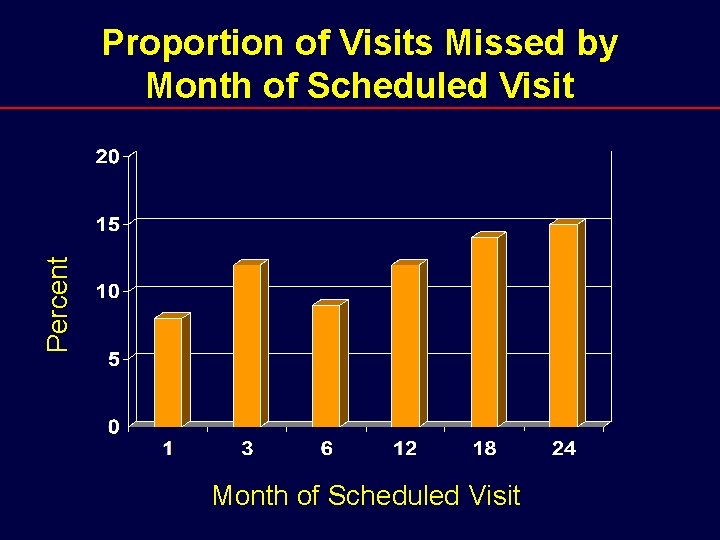
Percent Proportion of Visits Missed by Month of Scheduled Visit

Percent

Percent

Progress to July 17, 2006 · Recruitment was completed Sept 6, 2005 · 50% of men are in 18 -20 year age group. – 3. 6% of 18 -20 year-olds are HIV-infected – 11. 3% of 21 -24 year-olds are HIV-infected · 1, 334 circumcision procedures completed (95. 9%) · 69 in the treatment arm were not circumcised within six weeks (representing 5. 0% crossover). · 13 men have crossed over from control to circumcision arm (0. 9%).

Adverse Events · 27 AEs (1. 7%) definitely, probably or possibly related to the procedure: – 10 delayed healing or partially disrupted wounds (0. 7%) – 6 post-operative wound infections (0. 4%) – 4 bleeding episodes (0. 3%) – 7 (0. 5%) other events (2 excessive swelling; 1 anesthetic reaction; 1 excessive pain; 1 pubic abscess related to surgical tape; 1 case of folliculitis; and 1 participant who reported erectile dysfunction).

Other Surgical Outcomes · Level of satisfaction – 99% of clients very satisfied – 0. 5% somewhat satisfied – 1 client somewhat dissatisfied – 0 clients very dissatisfied · At 30 days post-op – 10% of participants reported having had sex since their circumcisions. – 99% of men had resumed work – 83% within 3 days · At 90 days post-op visit – 65% reported having resumed sexual intercourse – 92% of female partners were very satisfied with the outcome, 5% were somewhat satisfied.

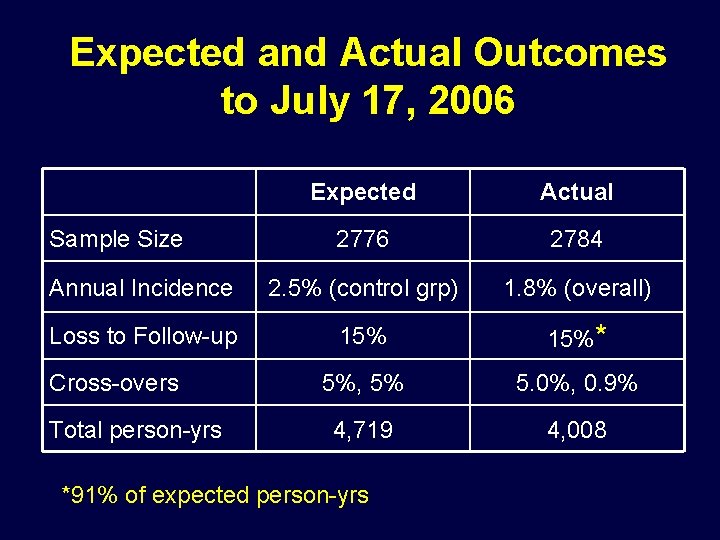
Expected and Actual Outcomes to July 17, 2006 Expected Actual 2776 2784 Annual Incidence 2. 5% (control grp) 1. 8% (overall) Loss to Follow-up 15%* 5%, 5% 5. 0%, 0. 9% 4, 719 4, 008 Sample Size Cross-overs Total person-yrs *91% of expected person-yrs

KEY EVENTS · June 29, 2005 – First Interim Analysis presented to DSMB. Instructed by DSMB to continue. · September, 2005 – Target enrollment of 2784 completed. · June 27, 2006 – Second interim analysis presented to DSMB. Instructed to continue. · September, 2007 – Completion of trial

 Biblical circumcision vs modern circumcision
Biblical circumcision vs modern circumcision Advantage of randomized controlled trial
Advantage of randomized controlled trial Male circumcision
Male circumcision Male circumcision
Male circumcision Male circumcision
Male circumcision The first trial of a controlled experiment allows
The first trial of a controlled experiment allows Circumcision
Circumcision Circumcision
Circumcision Pure heart scripture
Pure heart scripture Circumcised
Circumcised Disadvantages of circumcision
Disadvantages of circumcision Circumcision
Circumcision Ptsd circumcision
Ptsd circumcision Circumcision
Circumcision Prolapsplastik
Prolapsplastik Circumcision
Circumcision Statistical model for crd
Statistical model for crd Completely randomized design
Completely randomized design Rbd experimental design
Rbd experimental design Nivolumab pronounce
Nivolumab pronounce