A case of refractory severe steroiddependent asthma Please




















- Slides: 34

A case of refractory, severe, steroid-dependent asthma Please help! Bruce S. Bochner, M. D.

• 24 y/o AA female referred in 2/99 from southern Maryland for evaluation and management of uncontrolled asthma • At the time, 20 weeks pregnant (G 5, P 4) • Last two pregnancies were complicated by uncontrolled asthma and oral steroid use throughout the pregnancy • H/O asthma since age 12, frequent episodes of wheezing & cough without any obvious triggers or seasonal pattern • Review of accompanying records revealed that her FEV 1 can range from 30% to 80% predicted on any given visit

• Early on, exacerbations 1 x/yr, necessitating ER visits • Initially treated with Cromolyn, Vanceril and Albuterol • Since 1992, worsening asthma, increased ER visits and for 1998 at least 6 hospitalizations • In 1992, found to have multiple positive skin tests, tried on Im. Tx w/o improvement; in fact, exacerbations of wheezing with most shots • Frequent courses of antibiotics for bronchitis or sinusitis

• At the time of her 2/99 visit: – Daily nocturnal symptoms – Wheezing with minimal activity – Normal CXR – managed with Prednisone 30 mg q. AM, Flovent 110 2 puffs BID, Serevent 2 puffs BID, Alupent 2 puffs q 3 h and nebs PRN, Atrovent 4 puffs BID, Accolate 20 mg BID, and Cromolyn q 3 h

• Drug allergy Hx: acute rashes from Penicillin, Codeine, Ceclor; Erythromycin caused GI upset • Environ. Hx: Born and raised in MD, lives in a separate home, no pets • Family Hx: All of her four kids (two different fathers) have asthma; current pregnancy is with a third father

• PE: – Vitals: BP 105/66, P 112, RR 18, Wt 168 lbs, peak flow best effort 130 liters/min – GEN: Mild Cushingoid facies, no rashes – HEENT: Nasal exam normal, no lymphadenopathy or thyromegaly – LUNGS: Diffuse expiratory wheezing and prolonged expiratory phase; sounds were in chest but not neck – HEART: Normal S 1, S 2. – EXTREMITIES: No peripheral edema

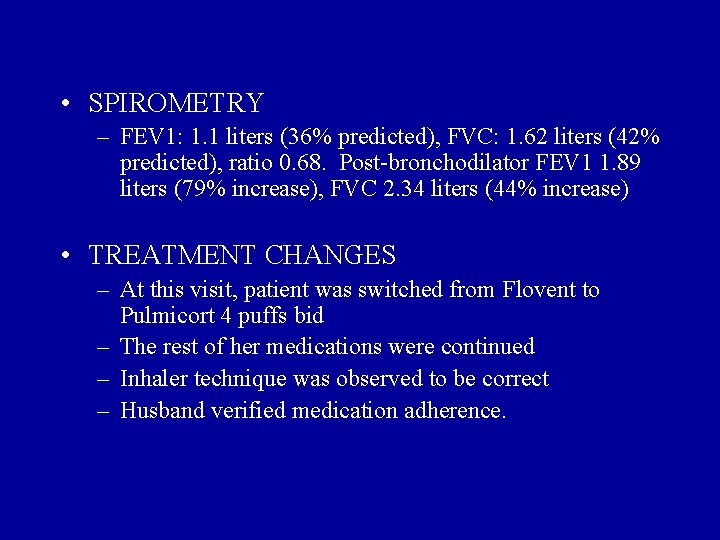
• SPIROMETRY – FEV 1: 1. 1 liters (36% predicted), FVC: 1. 62 liters (42% predicted), ratio 0. 68. Post-bronchodilator FEV 1 1. 89 liters (79% increase), FVC 2. 34 liters (44% increase) • TREATMENT CHANGES – At this visit, patient was switched from Flovent to Pulmicort 4 puffs bid – The rest of her medications were continued – Inhaler technique was observed to be correct – Husband verified medication adherence.

• Delivered the baby on continuous nebs. Baby and Mom did fine. 5 weeks postpartum admitted to Hopkins Bayview for 5 days for worsening SOB, wheezing and leg pain • On admission, wheezing; PEF 100 liters/min • V/Q scan and leg dopplers normal • FEV 1 28% predicted; flow-volume loops normal • CT scan of sinuses revealed pan-sinusitis • 24 -hr p. H probe documented significant GERD • Discharged on 24 -day steroid taper with markedly improved lung function at discharge; started on antibiotics and Prilosec

• Since 2000, multiple ER visits – two prolonged intubations in 2000 and 2001 • 2000: complicated by full respiratory arrest and persistent doll’s eyes • 2001: complicated by bilateral pneumothoraces requiring chest tubes and a DVT; s/p IVC filter • Multiple meds tried in 2000 -2001 included Advair, Pulmicort respules, Theophylline, and Methotrexate. None had a significant impact on our ability to taper oral steroids.

• In 10/01, sent for an outpatient evaluation by me to National Jewish (made possible through philanthropic help from NJC, AAFA and her local church) with dx of severe, labile steroid-dependent asthma • Diagnosis quickly confirmed when she required admission for worsening SOB and wheezing

• • • Skin tests positive to dust mites, grasses, alternaria Alpha-1 antitrypsin: normal CF genotyping: normal No peripheral blood eosinophilia Total Ig. E: 123 IU/ml Chest CT: no interstitial disease Bone densitometry: normal Sinus CT: mild sinusitis Oral steroid kinetics normal

• Seen by Drs. Barry Make and Sally Wenzel • After stabilization with IV steroids and nebs, underwent bronchoscopy • Found to have some collapsibility of her larynx with exhalation which they felt would be helped with CPAP • Sleep study found sleep apnea for which CPAP was also recommended

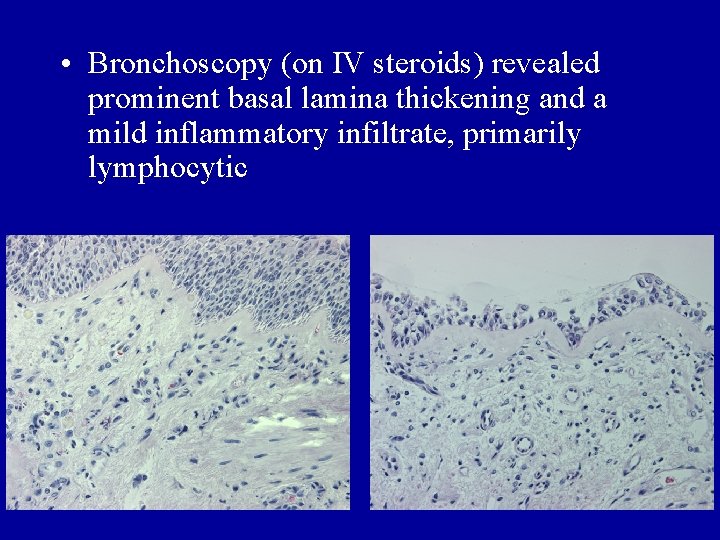
• Bronchoscopy (on IV steroids) revealed prominent basal lamina thickening and a mild inflammatory infiltrate, primarily lymphocytic

• After 3 weeks, sent back to Baltimore on the following regimen: – – – Serevent 3 puffs q 12 QVAR 6 puffs bid Atrovent 4 puffs qid Uniphyl 400 mg qhs Singulair 10 mg qhs Zyflo 600 mg qid Prilosec 40 mg qd Supplemental Calcium Prednisone 40 mg q am, 20 mg q afternoon Nasonex 1 spray bid CPAP

• Within 2 months, back to pre-Denver management • 2002 to 2003 – Managed primarily with Prednisone (40 -80 mg/day), Prilosec and Albuterol – Extremely Cushingoid; now weighs 240 lbs – Tried Xopenex w/o any additional benefit • September 2003 – Started Xolair one vial q month (completely covered by her insurer) – Still had ER visits but no hospitalizations while on Xolair – Despite this, after seven months, Prenisone, q 3 h albuteral requirements and FEV 1 remained unchanged – She became frustrated, so we discussed other options (Enbrel) and stopped Xolair

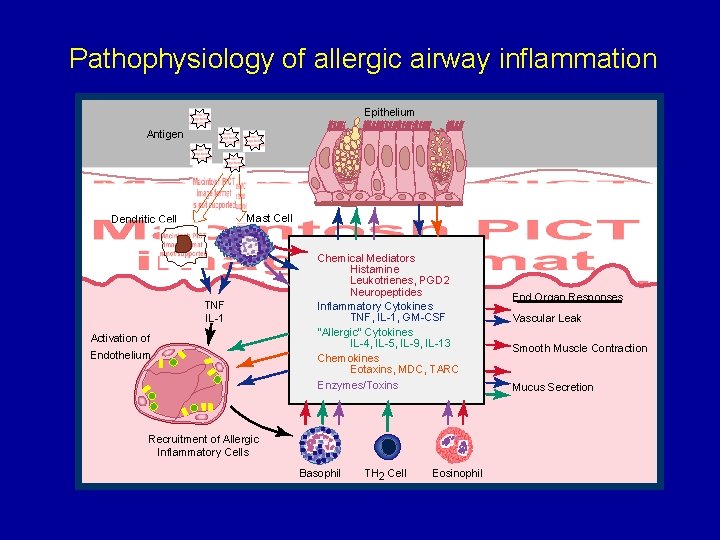
Pathophysiology of allergic airway inflammation Epithelium Antigen Mast Cell Dendritic Cell TNF IL-1 Activation of Endothelium Chemical Mediators Histamine Leukotrienes, PGD 2 Neuropeptides Inflammatory Cytokines TNF, IL-1, GM-CSF "Allergic" Cytokines IL-4, IL-5, IL-9, IL-13 Chemokines Eotaxins, MDC, TARC Enzymes/Toxins Recruitment of Allergic Inflammatory Cells Basophil TH 2 Cell Eosinophil End Organ Responses Vascular Leak Smooth Muscle Contraction Mucus Secretion

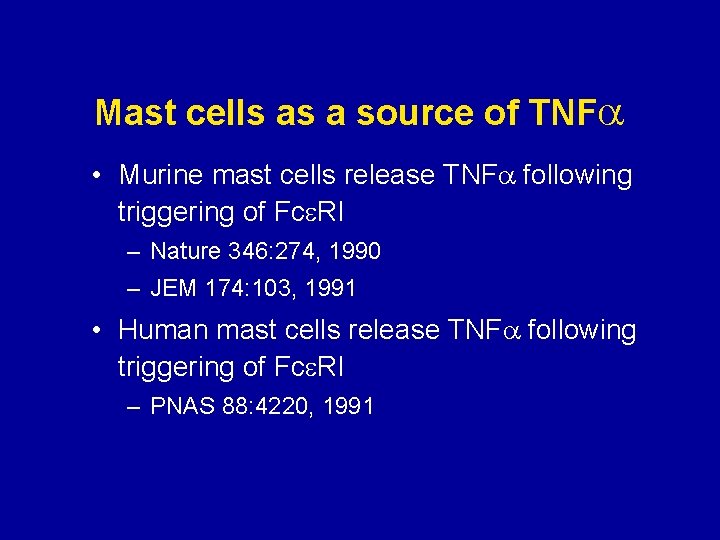
Mast cells as a source of TNF • Murine mast cells release TNF following triggering of Fce. RI – Nature 346: 274, 1990 – JEM 174: 103, 1991 • Human mast cells release TNF following triggering of Fce. RI – PNAS 88: 4220, 1991

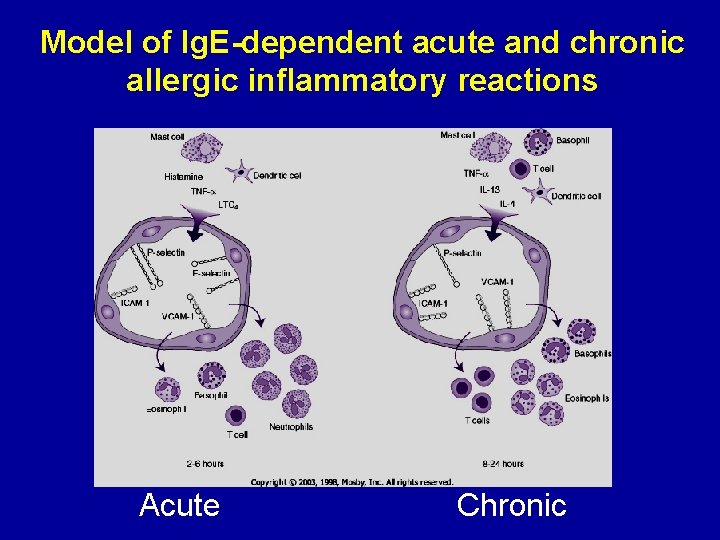
Model of Ig. E-dependent acute and chronic allergic inflammatory reactions Acute Chronic

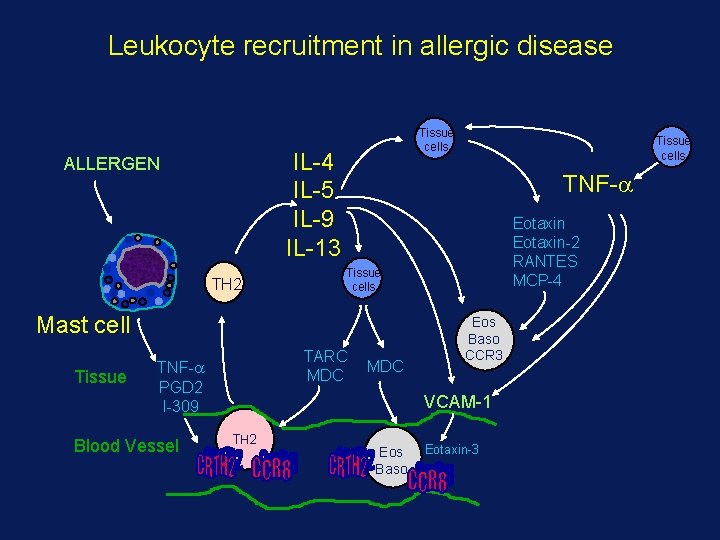
Leukocyte recruitment in allergic disease Tissue cells IL-4 IL-5 IL-9 IL-13 ALLERGEN TH 2 TNF- Tissue Blood Vessel Eotaxin-2 RANTES MCP-4 Tissue cells Mast cell TARC MDC TNF- PGD 2 I-309 Tissue cells MDC Eos Baso CCR 3 VCAM-1 TH 2 Eos Baso Eotaxin-3

Soluble Tumour Necrosis Factor Alpha (TNF-a) Receptor (Enbrel) as an Effective Therapeutic Strategy in Chronic Severe Asthma Babu KS, Arshad SH, Howarth PH, Chauhan AJ, Bell EJ, Puddicombe S, Davies DE, Holgate ST Respiratory Cell & Molecular Biology Southampton University Hospital Southampton, UK JACI 2003 (abstract)

Study design • Open label, single center study • Subjects with chronic severe asthma on oral corticosteroids, high dose inhaled corticosteroids, salmeterol, and/or theophylline • 25 mg of Enbrel administered subcutaneous twice a week for 12 weeks

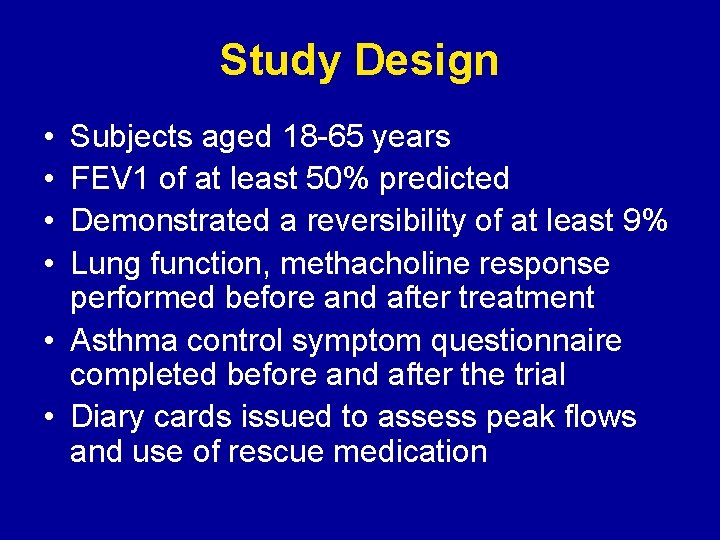
Study Design • • Subjects aged 18 -65 years FEV 1 of at least 50% predicted Demonstrated a reversibility of at least 9% Lung function, methacholine response performed before and after treatment • Asthma control symptom questionnaire completed before and after the trial • Diary cards issued to assess peak flows and use of rescue medication

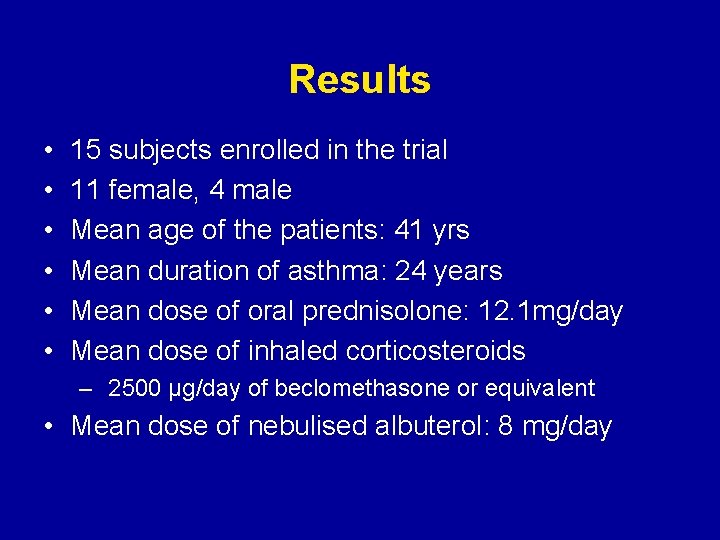
Results • • • 15 subjects enrolled in the trial 11 female, 4 male Mean age of the patients: 41 yrs Mean duration of asthma: 24 years Mean dose of oral prednisolone: 12. 1 mg/day Mean dose of inhaled corticosteroids – 2500 µg/day of beclomethasone or equivalent • Mean dose of nebulised albuterol: 8 mg/day

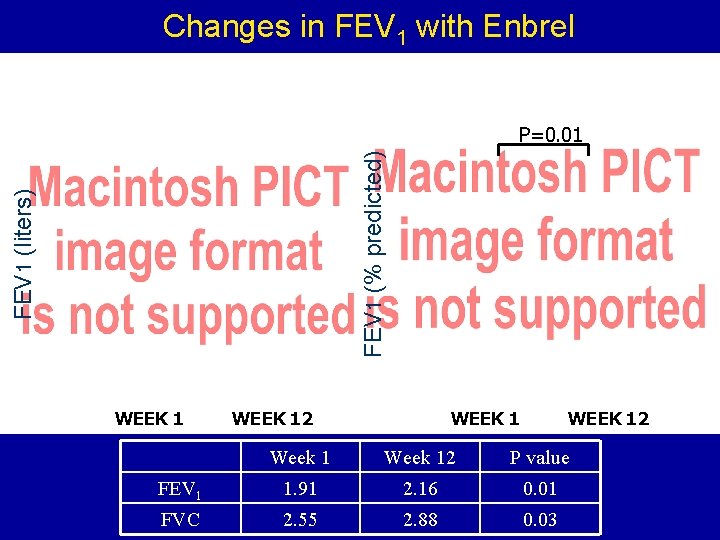
Changes in FEV 1 with Enbrel FEV 1 (liters) FEV 1 (% predicted) P=0. 01 WEEK 12 Week 12 P value FEV 1 1. 91 2. 16 0. 01 FVC 2. 55 2. 88 0. 03

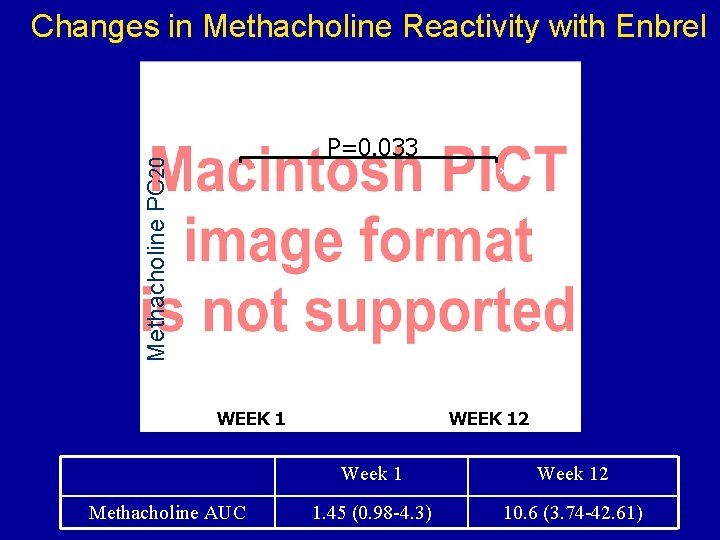
Changes in Methacholine Reactivity with Enbrel Methacholine PC 20 P=0. 033 * WEEK 1 Methacholine AUC WEEK 12 Week 12 1. 45 (0. 98 -4. 3) 10. 6 (3. 74 -42. 61)

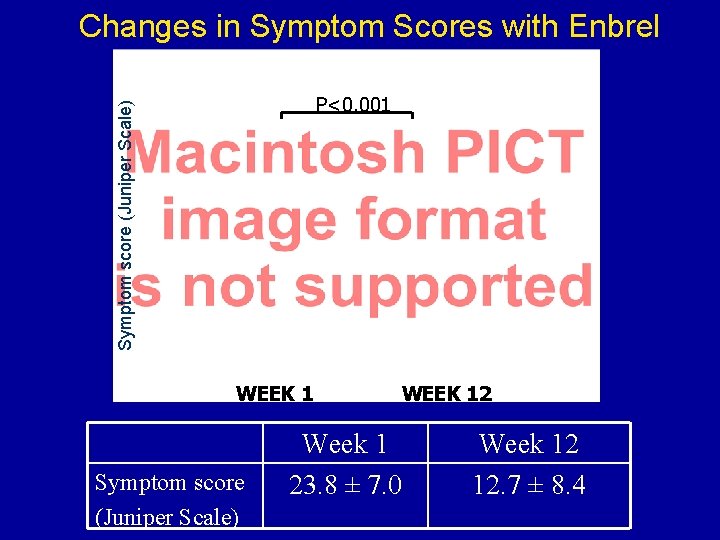
Changes in Symptom Scores with Enbrel Symptom score (Juniper Scale) P<0. 001 WEEK 1 Symptom score (Juniper Scale) WEEK 12 Week 1 23. 8 ± 7. 0 Week 12 12. 7 ± 8. 4

Adverse effects • Skin rashes (4) • Injection site reactions (4) • Respiratory tract infections (7) • Weakly positive ANA (3)

Conclusions Treatment with Enbrel in patients with chronic severe asthma: • Improves lung function (FEV 1, FEV 1/FVC, morning and evening PEF) • Markedly improves asthma control • Markedly improves airway hyperresponsiveness • Markedly reduces the need for rescue medications as all the subjects completely withdrew from their nebulised albuterol by the end of the study

• April - early June 2004 – Started Enbrel 25 mg sq twice weekly (completely covered by her insurer) after PPD was negative; husband trained on administration technique – Two weeks later, she was admitted for an asthma exacerbation associated with nausea, fatigue, myalgias and unexplained fevers to 102° despite Enbrel and prednisone; discovered Prilosec had been stopped – Infectious workup unrevealing; IV steroids given – Enbrel dosing held for 2 weeks, fever resolved – Enbrel restarted and 1 week later she was admitted for another asthma exacerbation – Enbrel discontinued

• June 21: planned to restart Xolair but got admitted again • Discharged June 22 • Seen June 23 – FEV 1 60%; FVC 93% – Diffuse wheezing on Prednisone 80 mg – Restarted Xolair 300 mg q 4 weeks – Restarted Serevent diskus 1 puff BID • What next? ?

Our ongoing work on TNF and allergic inflammation • There is a tissue-specific pattern of chemokines/cytokines/adhesion molecules involved in human allergic inflammation • This pattern is TNF- dependent • The primary source of TNF- released in human allergic inflammation is the mast cell

Etanercept in late phase cutaneous allergic inflammation: study overview • Randomized DBPC Trial • To evaluate effects of etanercept (Enbrel) on cutaneous allergen LPR in 10 perennial allergic rhinitis/dust mite sensitive patients • 15 visits to JHAAC over 8. 5 wks • Lead investigators: Lisa Beck, Ed Conner, Bruce Bochner

Study Purpose • To evaluate the clinical effects of etanercept on cutaneous allergen challenge late phase responses • To evaluate the effects of etanercept on the allergen dose response • To characterize a variety of biomarkers in the cutaneous late phase responses • To assess limited pharmacokinetic data of etanercept in the serum and nasal washings

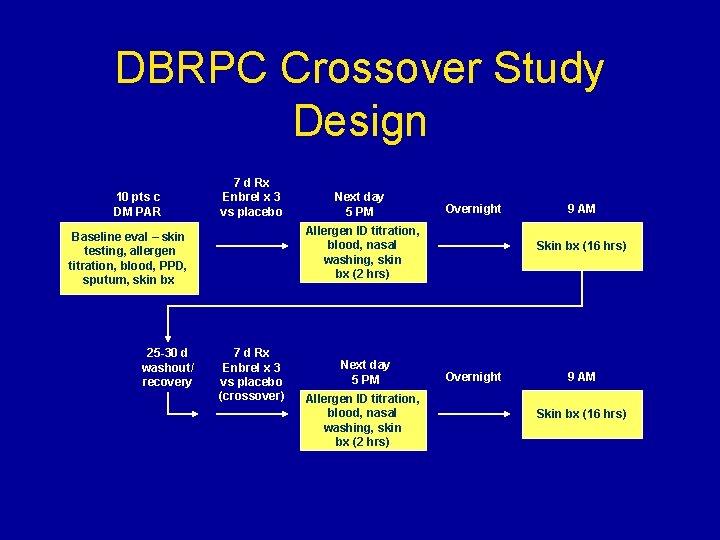
DBRPC Crossover Study Design 10 pts c DM PAR 7 d Rx Enbrel x 3 vs placebo Overnight Allergen ID titration, blood, nasal washing, skin bx (2 hrs) Baseline eval – skin testing, allergen titration, blood, PPD, sputum, skin bx 25 -30 d washout/ recovery Next day 5 PM 7 d Rx Enbrel x 3 vs placebo (crossover) Next day 5 PM Allergen ID titration, blood, nasal washing, skin bx (2 hrs) 9 AM Skin bx (16 hrs) Overnight 9 AM Skin bx (16 hrs)
 Is hyperpolarization the same as refractory period
Is hyperpolarization the same as refractory period A&p flix activity: resting membrane potential
A&p flix activity: resting membrane potential Asthma index
Asthma index Severe asthma treatment
Severe asthma treatment Mild moderate severe asthma exacerbation
Mild moderate severe asthma exacerbation Unmet needs in severe asthma
Unmet needs in severe asthma Etiology of bronchial asthma
Etiology of bronchial asthma International severe asthma registry
International severe asthma registry Pathophysiology definition
Pathophysiology definition Malnutrition case
Malnutrition case Best case worst case average case
Best case worst case average case Raymond carver will you please be quiet please
Raymond carver will you please be quiet please Male refractory time by age
Male refractory time by age Refractory period neuron
Refractory period neuron Refractive media of the eyeball
Refractive media of the eyeball Refractory periodontitis
Refractory periodontitis Graded potential
Graded potential Refractory period men
Refractory period men Absolute refractory period
Absolute refractory period Supranormal refractory period
Supranormal refractory period Absolute refractory period
Absolute refractory period Female secondary sexual characteristics
Female secondary sexual characteristics Refractory period cardiac
Refractory period cardiac Refractory shock
Refractory shock Biljana gjoneska
Biljana gjoneska Relative refractory period
Relative refractory period Refractory period men
Refractory period men Neutral refractory material factory
Neutral refractory material factory Refractory castable
Refractory castable Lighten refractory linings
Lighten refractory linings Smooth muscle fibres are fusiform and syncytial
Smooth muscle fibres are fusiform and syncytial Refractory period men
Refractory period men Refractory period heart
Refractory period heart Cool vs cold
Cool vs cold Membrane transport
Membrane transport