8 5 The Helmholtz Function The change in




- Slides: 14

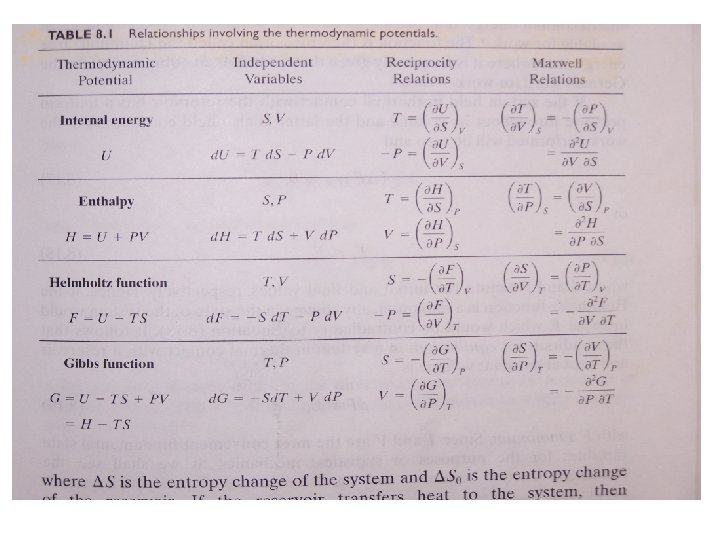
8. 5 The Helmholtz Function • The change in internal energy is the heat flow in an isochoric reversible process. • The change in enthalpy H is the heat flow in an isobaric reversible process. • The change in the Helmholtz function F in an isothermal reversible process is the work done on or by the system. • The decrease in F equals the maximum energy that can be made available for work.

8. 6 The Gibbs Function • Based on the second law of thermodynamics d. Q ≤ T∆S with d. Q = ∆U + P ∆V • Combine the above expressions ∆U + P ∆V ≤ T∆S ∆U + P ∆V - T∆S ≤ 0 • Since G = U + PV –TS (∆G)T, P≤ 0 at constant T and P or G f ≤ Gi • Gibbs function decreases in a process until a minimum is reach, i. e. equilibrium point. • Note that T and P need not to be constant throughout the process, they only need to have the same initial and final values.

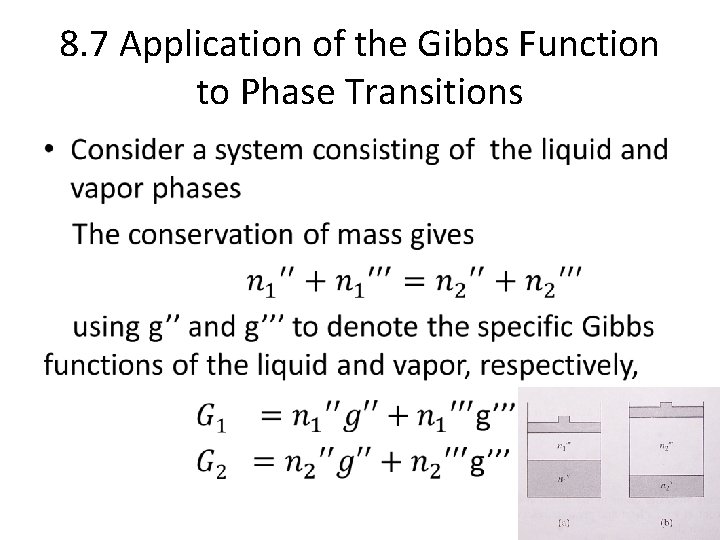
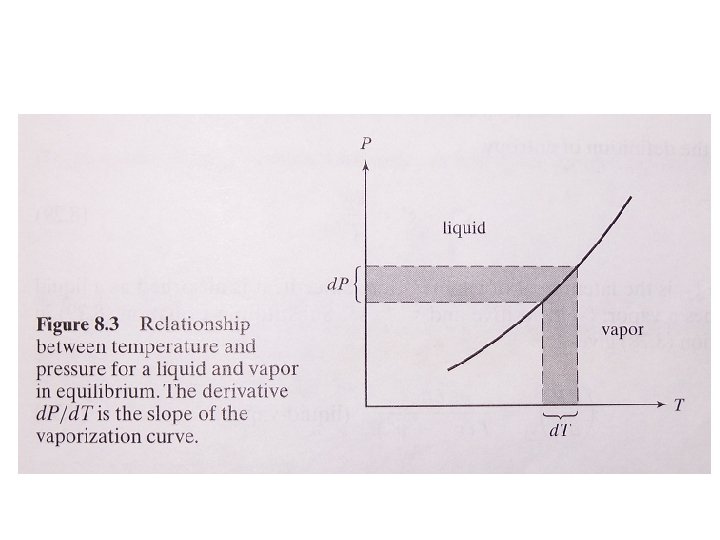
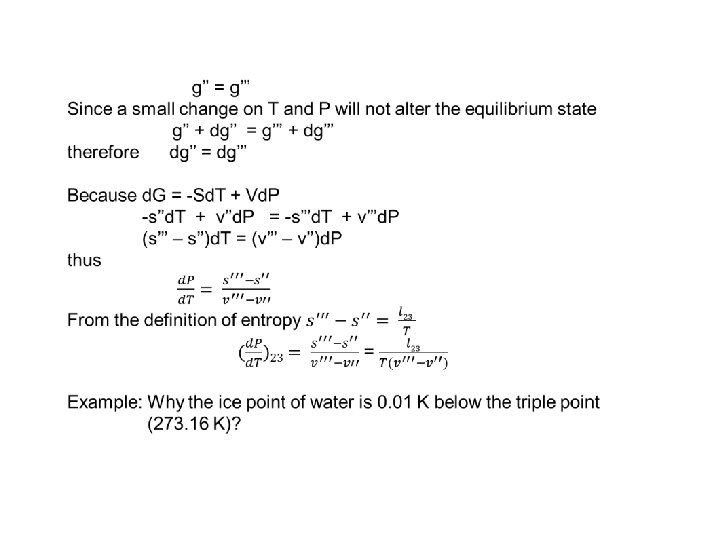
8. 7 Application of the Gibbs Function to Phase Transitions •



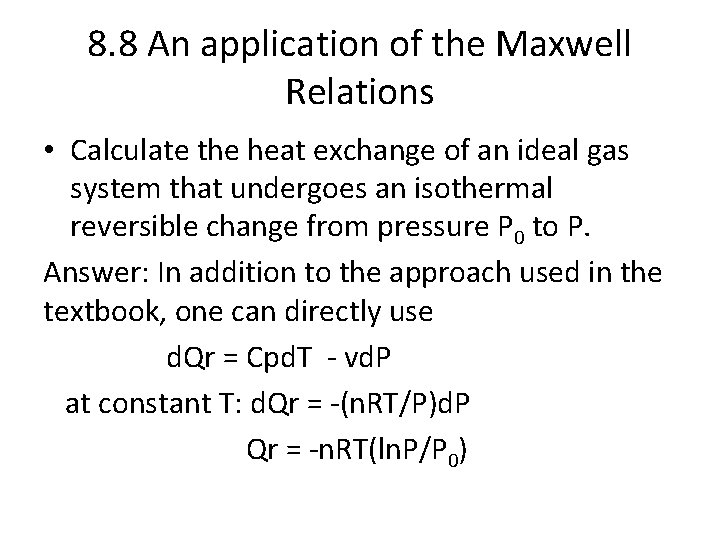
8. 8 An application of the Maxwell Relations • Calculate the heat exchange of an ideal gas system that undergoes an isothermal reversible change from pressure P 0 to P. Answer: In addition to the approach used in the textbook, one can directly use d. Qr = Cpd. T - vd. P at constant T: d. Qr = -(n. RT/P)d. P Qr = -n. RT(ln. P/P 0)


Chapter 9 The Chemical Potential and Open Systems

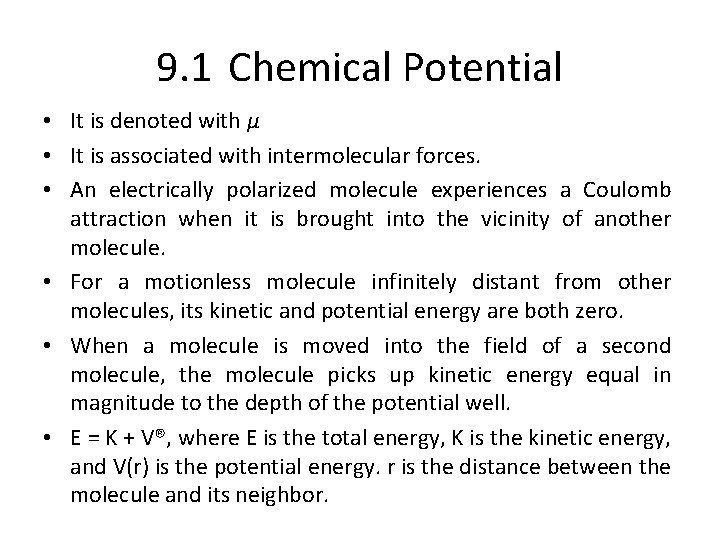
9. 1 Chemical Potential • It is denoted with µ • It is associated with intermolecular forces. • An electrically polarized molecule experiences a Coulomb attraction when it is brought into the vicinity of another molecule. • For a motionless molecule infinitely distant from other molecules, its kinetic and potential energy are both zero. • When a molecule is moved into the field of a second molecule, the molecule picks up kinetic energy equal in magnitude to the depth of the potential well. • E = K + V®, where E is the total energy, K is the kinetic energy, and V(r) is the potential energy. r is the distance between the molecule and its neighbor.

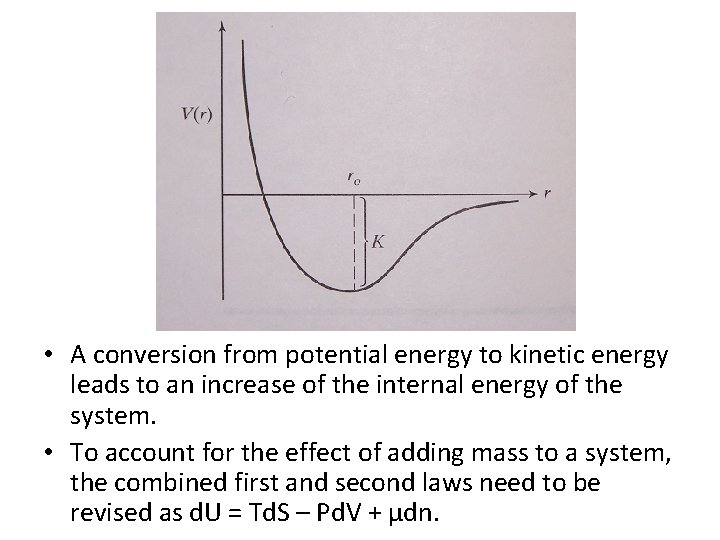
• A conversion from potential energy to kinetic energy leads to an increase of the internal energy of the system. • To account for the effect of adding mass to a system, the combined first and second laws need to be revised as d. U = Td. S – Pd. V + µdn.

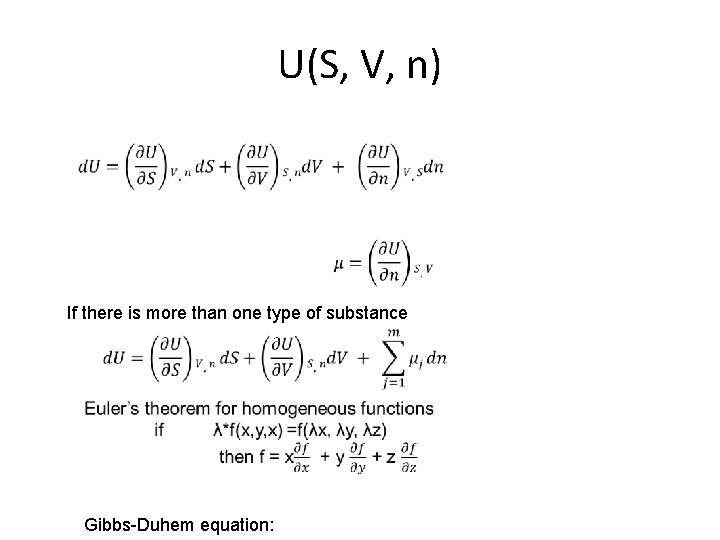
U(S, V, n) If there is more than one type of substance Gibbs-Duhem equation:

9. 2 The Phase equilibrium • The total system is enclosed in a rigid adibatic wall. • Conservation of mass. • Conservation of volume. • Conservation of energy. • At equilibrium the entropy of the total system is the maximum.

9. 3 The Gibbs Phase Rule 1. 2. 3. 4. Only one gaseous phase can exist because of diffusion. Several liquids can coexist in equilibrium if they are immiscible. Several solids can coexist. Only rarely do more than three phases of a given constituent coexist. For a system consists of k constitutes and π phases, the number of “degrees of freedom, called the variance f, is calculated with f = k – π + 2. for example: 1. A homogeneous fluid with one constituent 2. A homogeneous system consists of two constituents. 3. A system with two phases 4. A system with three phases.

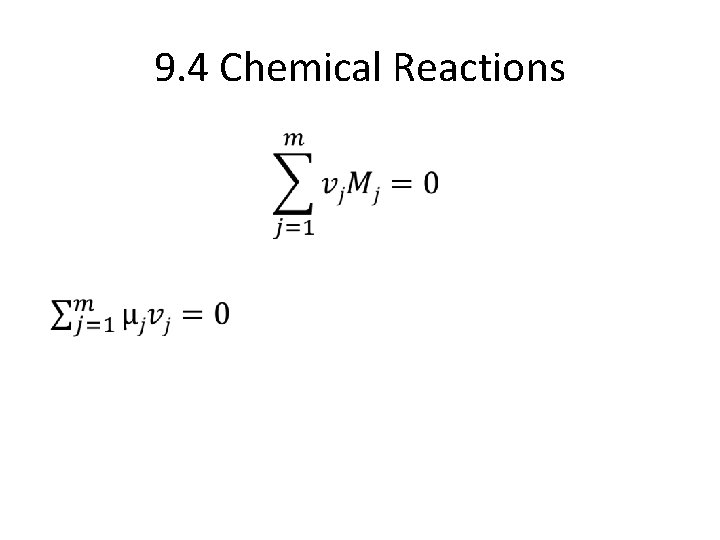
9. 4 Chemical Reactions •