8 3 The Dissolving Process Dissolving Compounds For






- Slides: 10

8. 3 The Dissolving Process

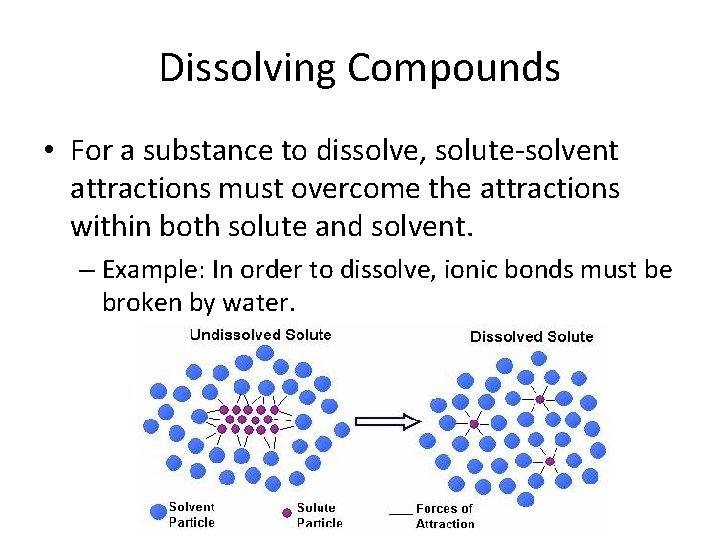
Dissolving Compounds • For a substance to dissolve, solute-solvent attractions must overcome the attractions within both solute and solvent. – Example: In order to dissolve, ionic bonds must be broken by water.

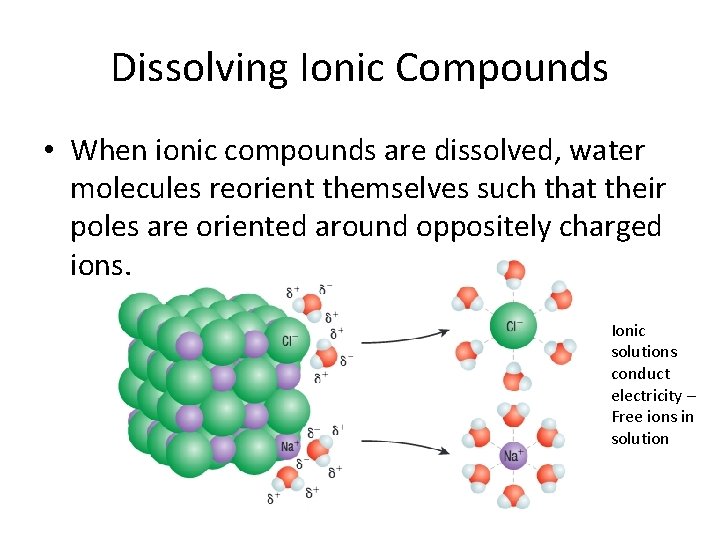
Dissolving Ionic Compounds • When ionic compounds are dissolved, water molecules reorient themselves such that their poles are oriented around oppositely charged ions. Ionic solutions conduct electricity – Free ions in solution

Dissolving Ionic Compounds • Hydration: the process in which ions are surrounded by water molecules • Dissociation: The process in which ions separate from ionic crystals, becoming individual ions. Na. Cl (s) Na+ (aq) + Cl- (aq) Na 2 CO 3 (s) 2 Na+ (aq) + CO 32 -(aq) Only ionic compounds undergo dissociation Water is not included as it does not undergo a chemical change

Writing Dissociation Equations Write dissociation equations for: – magnesium chloride – potassium phosphate – aluminum oxide – ammonium sulphate

Dissolving Molecular Compounds • Solubility of molecular compounds varies – glucose(C 6 H 12 O 6) and ethanol (C 2 H 5 OH) form solutions, while oil does not. • Miscible: liquids that can mix to form a solution – ie: alcohol and water • Immiscible: liquids that can not mix to form a solution – ie: oil and water

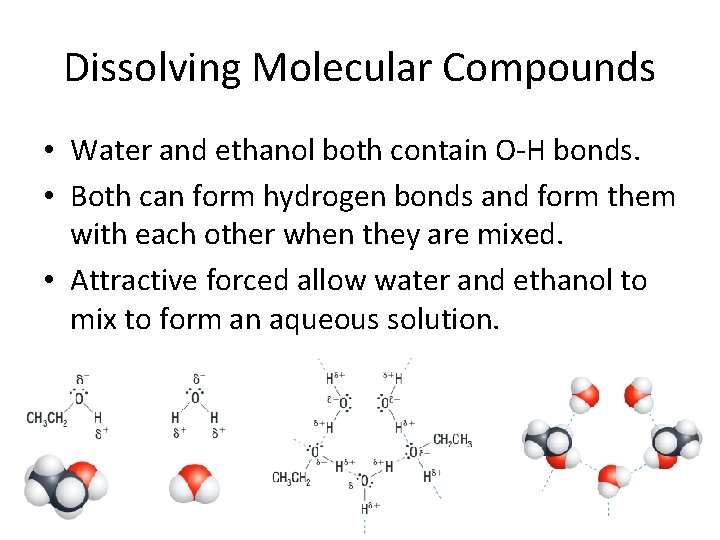
Dissolving Molecular Compounds • Water and ethanol both contain O-H bonds. • Both can form hydrogen bonds and form them with each other when they are mixed. • Attractive forced allow water and ethanol to mix to form an aqueous solution.

Like Dissolves Like • Ionic and polar compounds dissolve in polar solvents due to solute-solvent attractions. • Non-polar solutes do not dissolve in polar solvents – weak solute-solvent attractions • Non-polar solutes do dissolve in non-polar solvents. Oil and water do not mix!

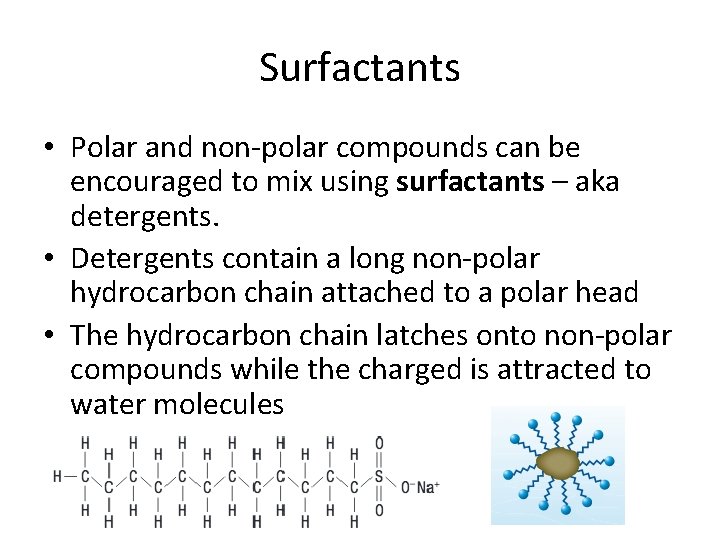
Surfactants • Polar and non-polar compounds can be encouraged to mix using surfactants – aka detergents. • Detergents contain a long non-polar hydrocarbon chain attached to a polar head • The hydrocarbon chain latches onto non-polar compounds while the charged is attracted to water molecules

Homework Read: Section 8. 3 Questions: Page 389 # 6, 8, 9, 10, 15,