2 2 ORGANIC MATTER ORGANIC MATTER Diederik Rousseau




- Slides: 19

- 2. 2 – ORGANIC MATTER

ORGANIC MATTER (Diederik Rousseau UNESCO-IHE Institute for Water Education Online Module Water Quality Assessment 2

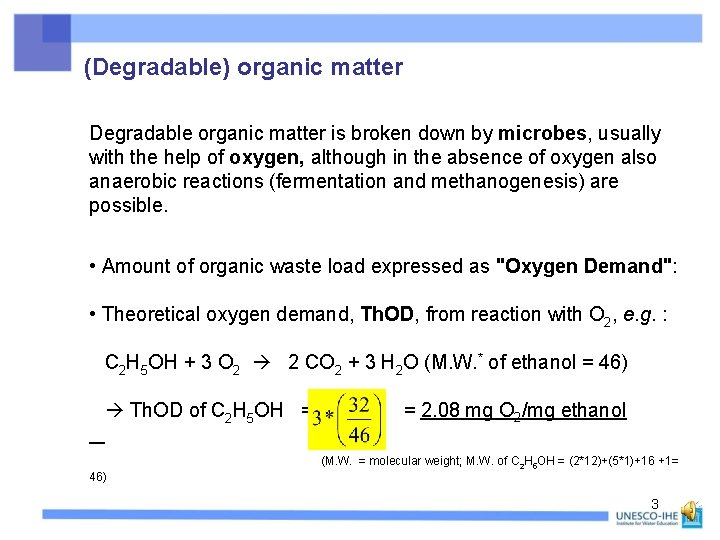
(Degradable) organic matter Degradable organic matter is broken down by microbes, usually with the help of oxygen, although in the absence of oxygen also anaerobic reactions (fermentation and methanogenesis) are possible. • Amount of organic waste load expressed as "Oxygen Demand": • Theoretical oxygen demand, Th. OD, from reaction with O 2, e. g. : C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O (M. W. * of ethanol = 46) Th. OD of C 2 H 5 OH = = 2. 08 mg O 2/mg ethanol (M. W. = molecular weight; M. W. of C 2 H 5 OH = (2*12)+(5*1)+16 +1= 46) 3

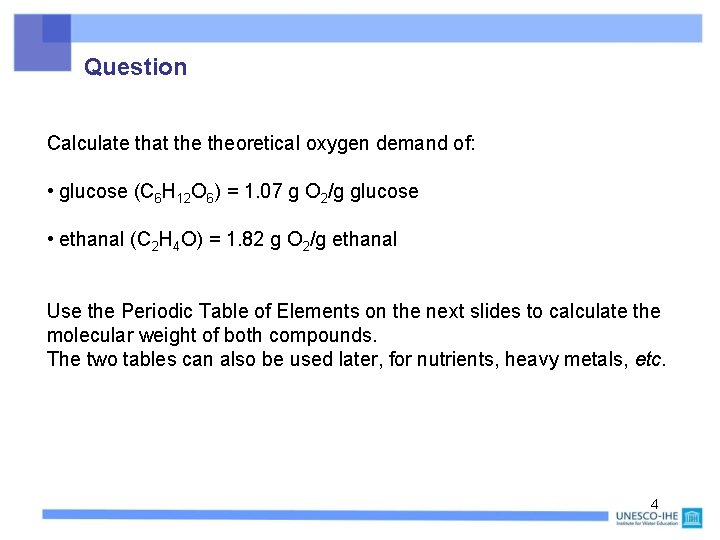
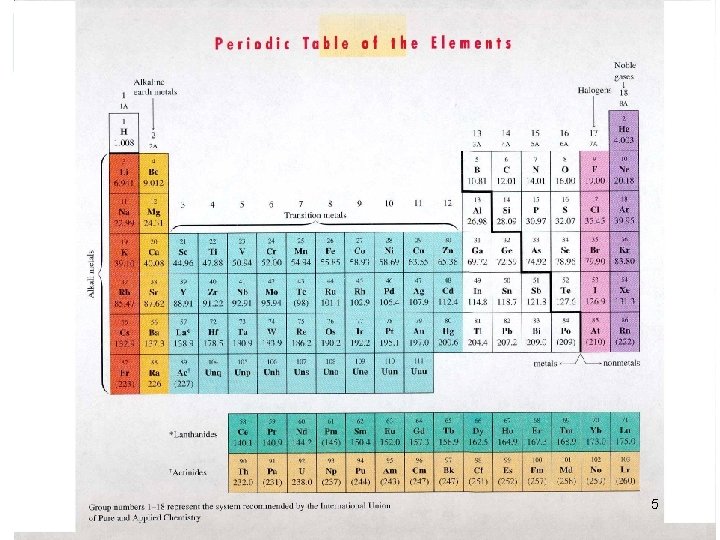
Question Calculate that theoretical oxygen demand of: • glucose (C 6 H 12 O 6) = 1. 07 g O 2/g glucose • ethanal (C 2 H 4 O) = 1. 82 g O 2/g ethanal Use the Periodic Table of Elements on the next slides to calculate the molecular weight of both compounds. The two tables can also be used later, for nutrients, heavy metals, etc. 4

5

6

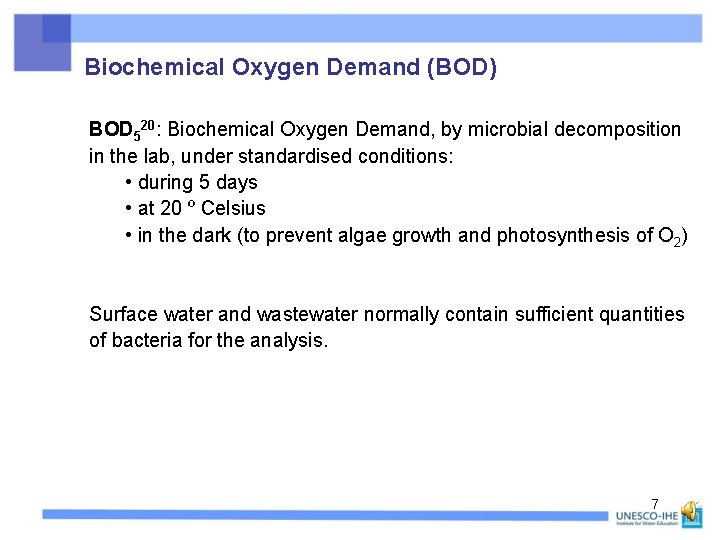
Biochemical Oxygen Demand (BOD) BOD 520: Biochemical Oxygen Demand, by microbial decomposition in the lab, under standardised conditions: • during 5 days • at 20 º Celsius • in the dark (to prevent algae growth and photosynthesis of O 2) Surface water and wastewater normally contain sufficient quantities of bacteria for the analysis. 7

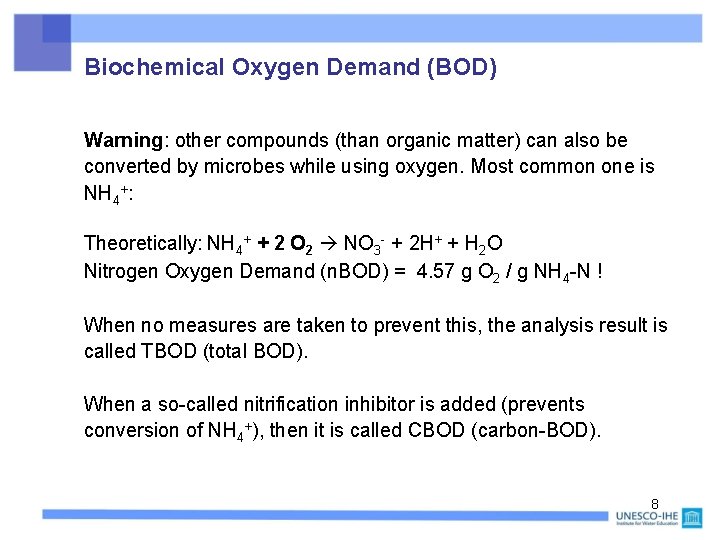
Biochemical Oxygen Demand (BOD) Warning: other compounds (than organic matter) can also be converted by microbes while using oxygen. Most common one is NH 4+: Theoretically: NH 4+ + 2 O 2 NO 3 - + 2 H+ + H 2 O Nitrogen Oxygen Demand (n. BOD) = 4. 57 g O 2 / g NH 4 -N ! When no measures are taken to prevent this, the analysis result is called TBOD (total BOD). When a so-called nitrification inhibitor is added (prevents conversion of NH 4+), then it is called CBOD (carbon-BOD). 8

INTERMEZZO: NH 4 -N or NH 4? In water quality monitoring we can express concentration as: • Based on the molecule, so mg NH 4/L (M. W. = 14 + 4 =18) (rounded off) • Based on the atom(s), so mg NH 4 -N/L (Atomic weight A. W. = 14) So a water quality of 1. 0 mg NH 4/L corresponds to 0. 78 mg NH 4 - N/L. Similarly: the Worlds Health Organization, WHO, guideline for nitrate in drinking water = 50 mg NO 3/L, equivalent to (14/62)*50 = 11. 3 mg NO 3 -N/L. It is highly recommendable to use the “Atoms system” (e. g. to make mass balances) Be very aware, in water quality data interpretation as well as in your own data reporting, of the way the results are expressed ! It’s a big source of errors! 9

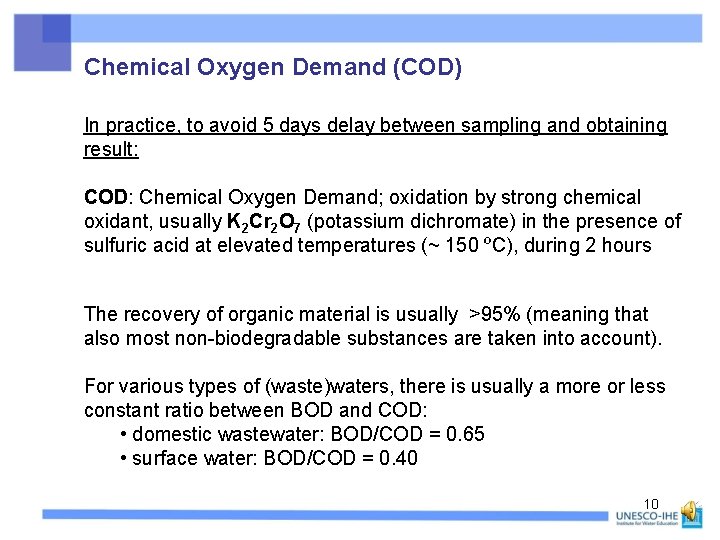
Chemical Oxygen Demand (COD) In practice, to avoid 5 days delay between sampling and obtaining result: COD: Chemical Oxygen Demand; oxidation by strong chemical oxidant, usually K 2 Cr 2 O 7 (potassium dichromate) in the presence of sulfuric acid at elevated temperatures (~ 150 ºC), during 2 hours The recovery of organic material is usually >95% (meaning that also most non-biodegradable substances are taken into account). For various types of (waste)waters, there is usually a more or less constant ratio between BOD and COD: • domestic wastewater: BOD/COD = 0. 65 • surface water: BOD/COD = 0. 40 10

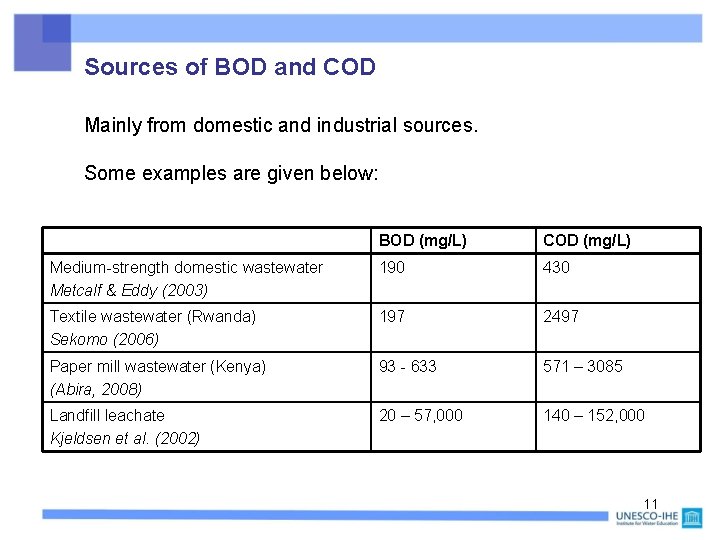
Sources of BOD and COD Mainly from domestic and industrial sources. Some examples are given below: BOD (mg/L) COD (mg/L) Medium-strength domestic wastewater Metcalf & Eddy (2003) 190 430 Textile wastewater (Rwanda) Sekomo (2006) 197 2497 Paper mill wastewater (Kenya) (Abira, 2008) 93 - 633 571 – 3085 Landfill leachate Kjeldsen et al. (2002) 20 – 57, 000 140 – 152, 000 11

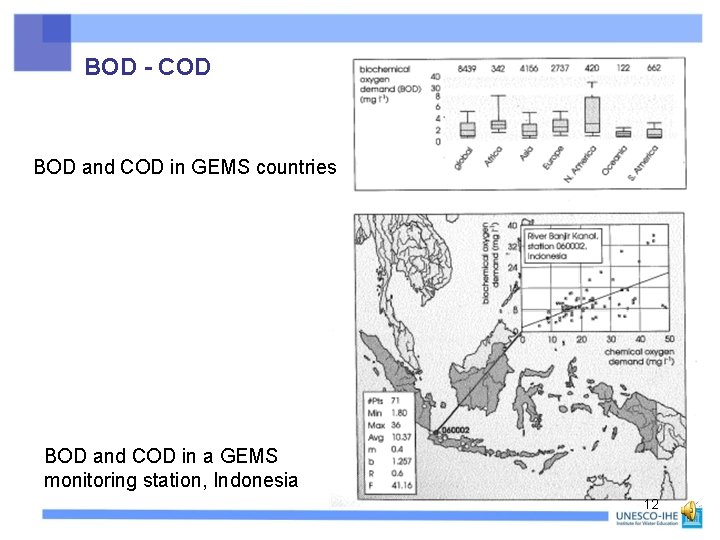
BOD - COD BOD and COD in GEMS countries BOD and COD in a GEMS monitoring station, Indonesia 12

BOD – COD 13

Assignment Have a look at the BOD / COD data for your own country at: http: //www. gemstat. org/ 14

Other related parameters • TOC – Total Organic Carbon • DOC – Dissolved Organic Carbon (see lecture notes) • Micro-organic pollutants (see Unit 4) 15

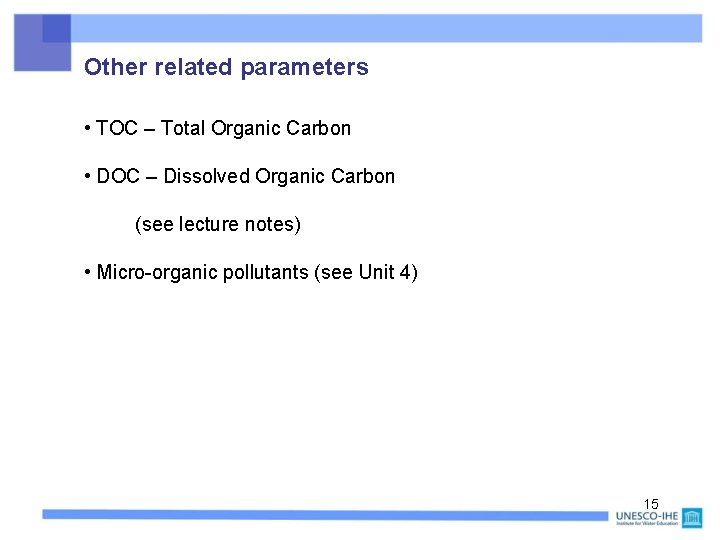
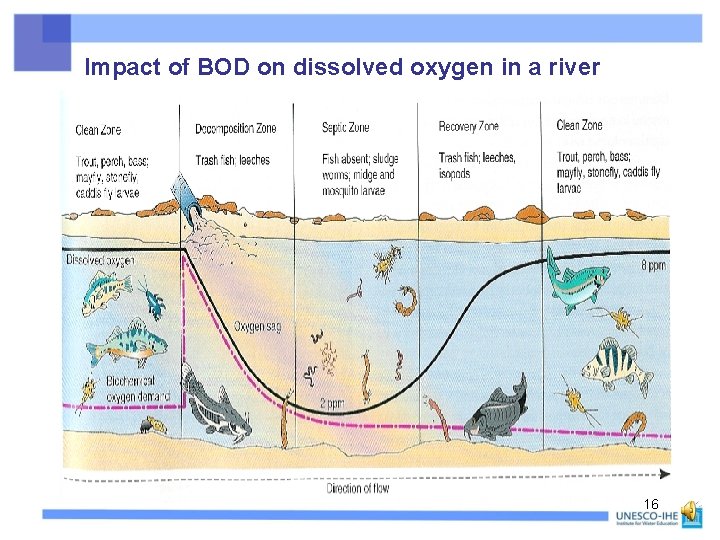
Impact of BOD on dissolved oxygen in a river 16

Impact of BOD on dissolved oxygen in a river See Unit 2. 6. : Modelling, for the general formula of the oxygen sag curve, for the minimum DO (“depth of oxygen sag is roughly proportional to BOD load”), and for the distance to reach the minimum 17

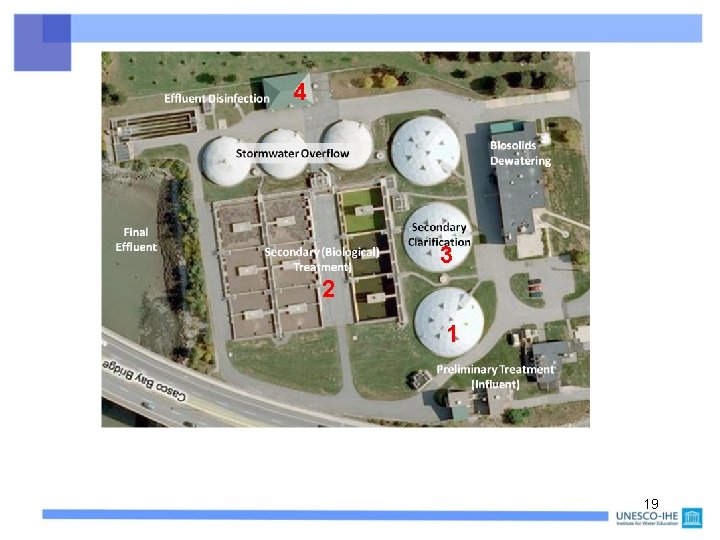
Wastewater treatment for removing of (see next slide): • Suspended solids in sewage (1) • CBOD, NBOD by microbes, under strong aeration (2) • Bacterial “sludge” (3) • Extra: Pathogens (4), e. g. by U. V. radiation • Also as extra (expensive!): nutrients (phosphate, nitrate, … see Course 2. 3. ) Cost recovery often: “the polluter pays” (households, factories, schools, . . . ) The Netherlands: BOD reduction from 1970 -2005: > 90% ! 18

4 3 2 1 19
 Theoretical oxygen demand
Theoretical oxygen demand Beukenlaan 1 breda
Beukenlaan 1 breda Stapel diederik
Stapel diederik Diederik stapel
Diederik stapel Value free research
Value free research Diederik stapel
Diederik stapel Diederik van wijk
Diederik van wijk Diederik den dekker
Diederik den dekker Mixture of weathered rock and organic matter
Mixture of weathered rock and organic matter Decomposition of organic matter equation
Decomposition of organic matter equation Partially decomposed organic matter in the soil is
Partially decomposed organic matter in the soil is Plantlike organisms that live on dead organic matter
Plantlike organisms that live on dead organic matter High organic matter soil
High organic matter soil Soil organic matter
Soil organic matter Contrato social hobbes
Contrato social hobbes Christophe rousseau
Christophe rousseau 1844 1910
1844 1910 Mvei
Mvei Dantova bárka
Dantova bárka Rousseau buon selvaggio
Rousseau buon selvaggio