13 5 The Effect of Temperature on Reaction




- Slides: 11

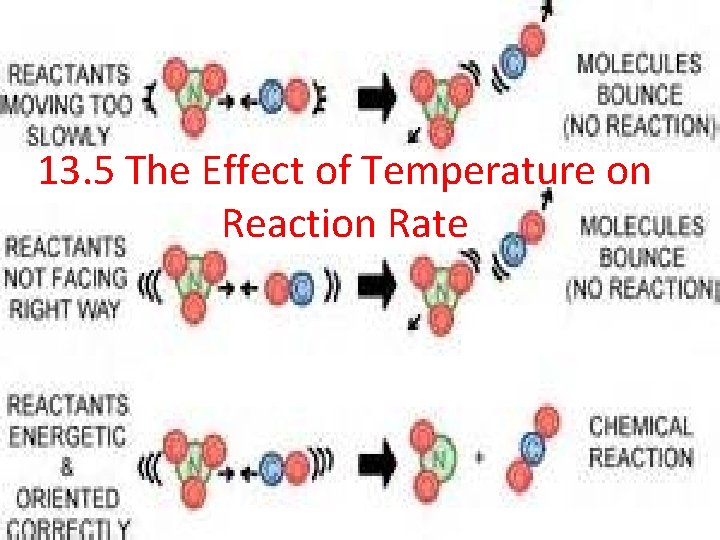
13. 5 The Effect of Temperature on Reaction Rate

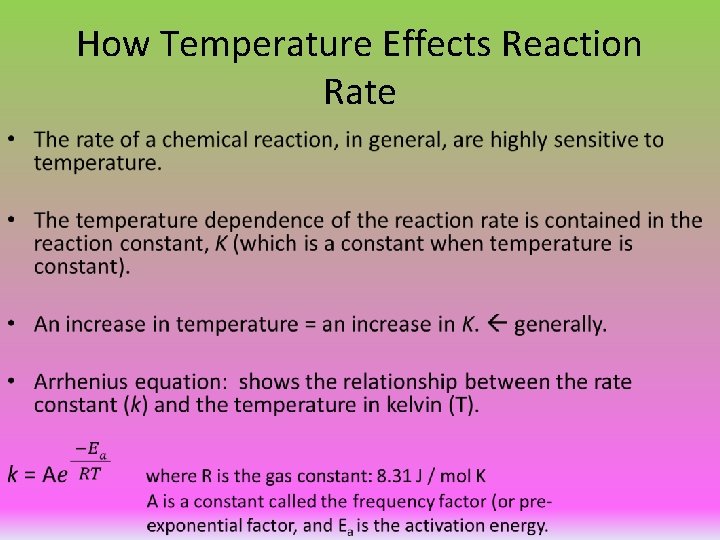
How Temperature Effects Reaction Rate •


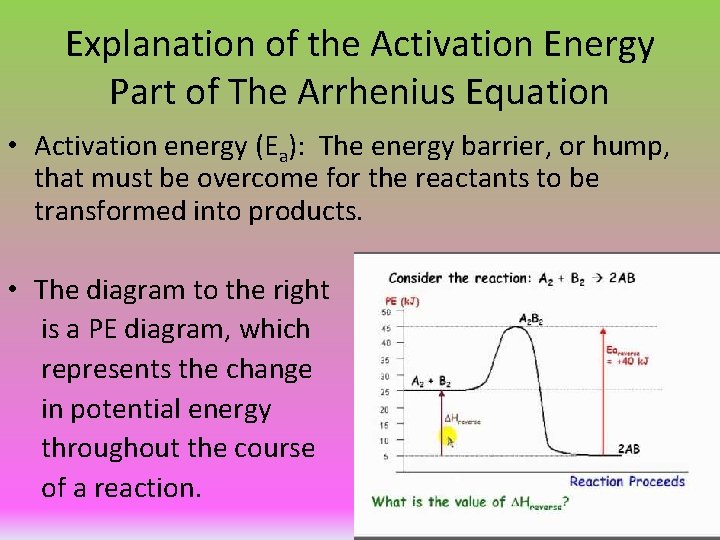
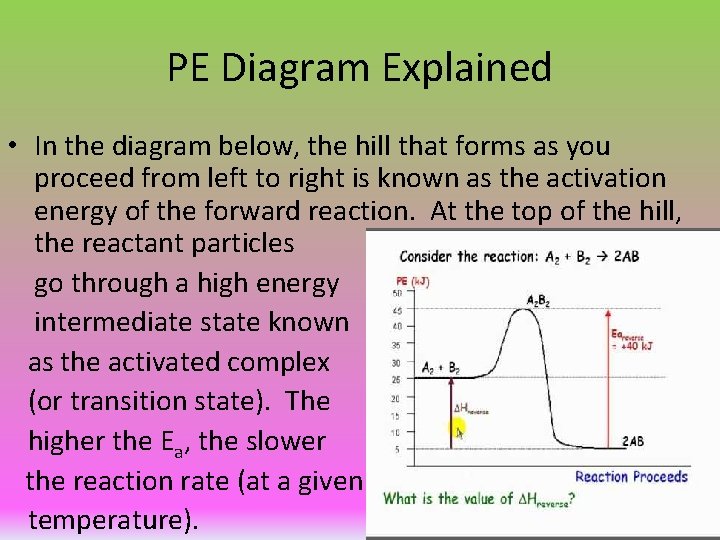
Explanation of the Activation Energy Part of The Arrhenius Equation • Activation energy (Ea): The energy barrier, or hump, that must be overcome for the reactants to be transformed into products. • The diagram to the right is a PE diagram, which represents the change in potential energy throughout the course of a reaction.

PE Diagram Explained • In the diagram below, the hill that forms as you proceed from left to right is known as the activation energy of the forward reaction. At the top of the hill, the reactant particles go through a high energy intermediate state known as the activated complex (or transition state). The higher the Ea, the slower the reaction rate (at a given temperature).

Frequency Factor and Exponential Factor • Frequency factor (A): the number of times that the reactants approach the activation barrier per unit time. • There are many instances when the particles collide and the reactant(s) approach the activation barrier, but most approaches are unsuccessful in overcoming the amount of energy necessary for the reaction to occur. • The exponential factor: a number between 0 and 1 that represents the fraction of particles that have enough energy to overcome the activation energy on a given approach. The exponential factor depends on both temperature and the activation energy of the reaction. • The rate constant k = frequency factor X exponential factor

Let’s Try a Practice Problem! Reaction A and reaction B have identical frequency factors. However, reaction B has a higher activation energy than reaction A. Which reaction has a greater rate constant at room temperature? Reaction A has a higher rate constant, since it has a lower activation energy; therefore the exponential factor is larger at any given temperature, making the rate constant larger. (With a larger rate constant and the same initial concentration, the rate will be faster).

The Collision Model: A Closer Look at Frequency Factor • In a collision model, a chemical reaction occurs after a sufficient energetic collision between two reactant particles. • In collision theory, each collision = an approach to the activation barrier of the reaction. So, the number of collisions of reacting particles should be the frequency factor, however it’s not that simple. • In most, if not all gas phase reactions, two factors have to be considered: 1. ) collision frequency, and 2. ) orientation factor.

The Collision Model: A Closer Look at Frequency Factor (continued) • The collision frequency: the number of collisions per unit time. (remember, both temperature and pressure affect the number of collisions made possible in the gas phase). • The orientation factor: a number usually between 0 and 1 that represents the number of collision with proper orientation that allows the reaction to occur. – A small orientation factor (one that approaches 0) means that the orientation requirements for this for the reaction are very stringent, that is, the molecule has to be aligned in a very specific way for the reaction to occur. • There also reaction where particles don’t even need to collide for a reaction to occur: example K(g) + Br 2(g) KBr(g) + Br(g). The transfer of electrons here can occur without a collision. This is known as a harpoon mechanism.

Let’s Try Another Practice Problem! Which reaction would you expect to have the smallest orientation factor? (a) H(g) + I(g) HI(g) (b) H 2(g) + I 2(g) 2 HI (g) (c) HCl(g) + HCl(g) H 2(g) + Cl 2(g), since here the reactants are assymetrical and must therefore collide in such a way that a hydrogen atom is in close proximity to another hydrogen atom. Therefore, we expect (c) to have the smallest orientation factor.

13. 5 pgs. 641 -642 #’s 57, 58, 71 and 72 Read 13. 6 -13. 7 pgs. 622 -632
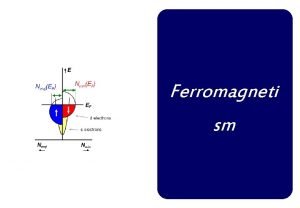
 Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Difference between curie temperature and neel temperature
Difference between curie temperature and neel temperature Ferromagnetis
Ferromagnetis Factors affecting the rate of chemical reaction temperature
Factors affecting the rate of chemical reaction temperature Chemical equation examples
Chemical equation examples Maxwell boltzmann distribution catalyst
Maxwell boltzmann distribution catalyst Equilibrium expressions
Equilibrium expressions In exothermic reaction temperature
In exothermic reaction temperature Exothermic reaction increase temperature equilibrium
Exothermic reaction increase temperature equilibrium Exothermic reaction increase temperature
Exothermic reaction increase temperature How to calculate activation energy
How to calculate activation energy Theory of solubility
Theory of solubility