1 st ANFEC Training workshop Royal University of








- Slides: 12

1 st ANFEC Training workshop Royal University of Phnom Penh On Environmental Contaminant Analysis 18 -20 December, 2012 at Royal University of Phnom Penh Organized by: Asian Network of Research on Food and Environment Contaminants (ANFEC) Supported by: Royal University of Phnom Penh, Cambodia International Science Programme, Uppsala University, Sweden.

Analysis of Arsenic By Silver Diethyldithiocarbamate Method Dr. Mohammad Arifur Rahman Department of Chemistry University of Dhaka-1000 Bangladesh rmarif@univdhaka. edu

Arsenic determination by the silver diethyldithiocarbamate method and the elimination of metal ion interferences: By Shingara S. Sandhu Reprints from the collection of the university of Michigan Library

A rapid colorimetric method for measuring arsenic concentrations in groundwater R. K. Dhara, b, c, ∗, Y. Zhenga, b, c, J. Rubenstonec, A. van Geenc a School of Earth and Environmental Sciences, NSB D-216, Queens College, City University of New. York, 65 -30 Kissena Building, Flushing, NY 11367, USA b Graduate School and University Center, City University of New. York, NY, USA c Lamont-Doherty Earth Observatory of Columbia University, Palisades, NY 10964, USA Received 5 May 2004; received in revised form 16 September 2004; accepted 16 September 2004

Spectrometric Method of Analysis Arsine (As. H 3) generation followed by complexation with silver –diehyldithiocarbamate [Ag-SCSN (C 2 H 5)2] solution: Arsenic reacts with a solution of Ag-DDTC, complex with morpholine in chloroform to form soluble red complex, which has an absorption maximum at 535 nm. Trivalent arsenic reacts with silver-diehyldithiocarbamate [Ag-SCSN(C 2 H 5)2] to form a red color complex, which absorbs at 535 nm. Ag-DDTC + As. H 3 → As-DDTC (red color) This is one of the standard and reliable method for arsenic determination in water at the ppb (parts per billion), g/L, level.

5 valence As ion (As 5+)+ (HCl, KI, Sn. Cl 2)→ 3 valence As ion (As 3+) By adding Zn to this solution, the arsenic is reduced further to arsenic hydride.

Preparation of Ag-DDTC Solution for Arsenic Adsorption The solution was prepared by dissolving 0. 75 g Ag-DDTC in a solution of 1 ml of morpholine dissolved in 100 ml of chloroform. Then it was kept in a round bottom quick fitted flask covering with aluminium foil and preserved at 4 degree in a refrigerator.

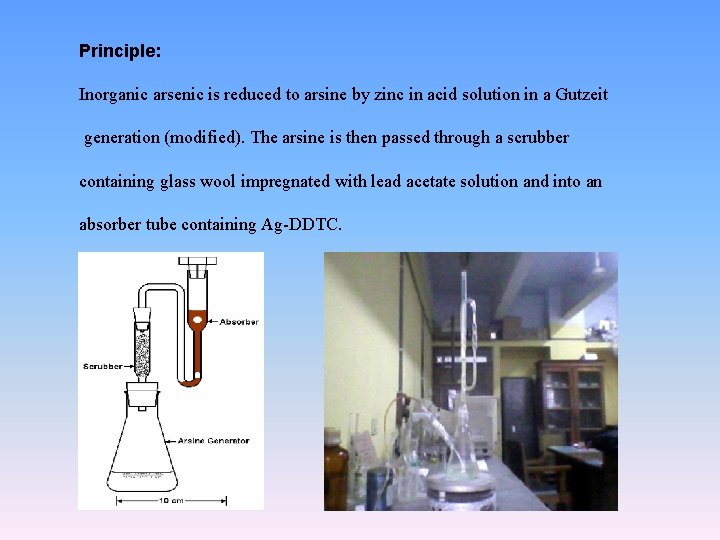
Principle: Inorganic arsenic is reduced to arsine by zinc in acid solution in a Gutzeit generation (modified). The arsine is then passed through a scrubber containing glass wool impregnated with lead acetate solution and into an absorber tube containing Ag-DDTC.

Preparation of necessary Solution and Reagent Preparation of Potassium Iodide Solution 15 g potassium Iodide (KI) was dissolved in 100 m. L distilled de-ionized water. Preparation of Stannous Chloride 10% 10 g stannous chloride (Sn. Cl 2. 2 H 2 O) was dissolved in 100 m. L distilled deionized water.

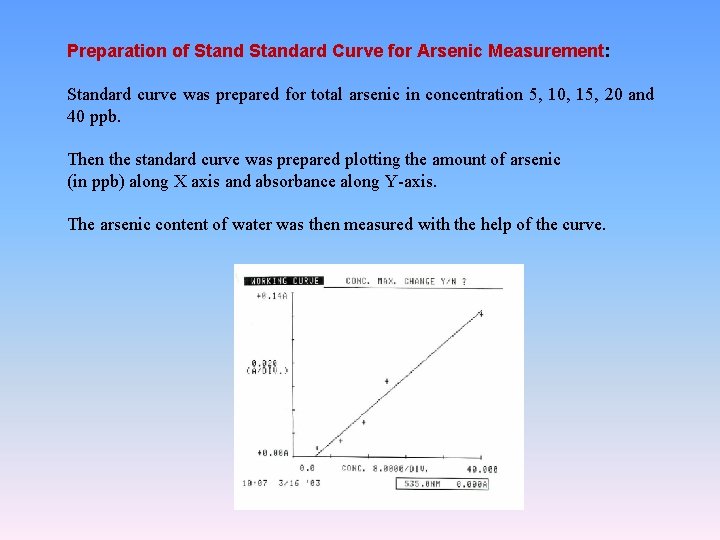
Preparation of Standard Curve for Arsenic Measurement: Standard curve was prepared for total arsenic in concentration 5, 10, 15, 20 and 40 ppb. Then the standard curve was prepared plotting the amount of arsenic (in ppb) along X axis and absorbance along Y-axis. The arsenic content of water was then measured with the help of the curve.

Procedure Treatment of Sample 50 m. L sample was taken into a clean generator bottle (quick fitted 100 ml conical flask) adding successively, 5 m. L 1: 1 HCl, 5 m. L KI (15%) solution and 8 drops Sn. Cl 2 reagent. Preparation of Scrubber and Absorber Cotton was impregnated in the scrubber with lead acetate solution and 4 m. L Ag. DDTC reagent was taken with a pipette in absorber tube. Arsenic Generation and Measurement Adding 1 g Zn-dust to generator, it was connected with scrubber absorber immediately. All connection must be fitted tightly. Allowing 30 min, for complete evolution of arsine and pouring solution absorber into a 1 cm cell, the absorbance was measured at 540 nm, using the reagent blank as the reference.
