Week 5 1 Create Kai A and Kai









- Slides: 11

Week 5

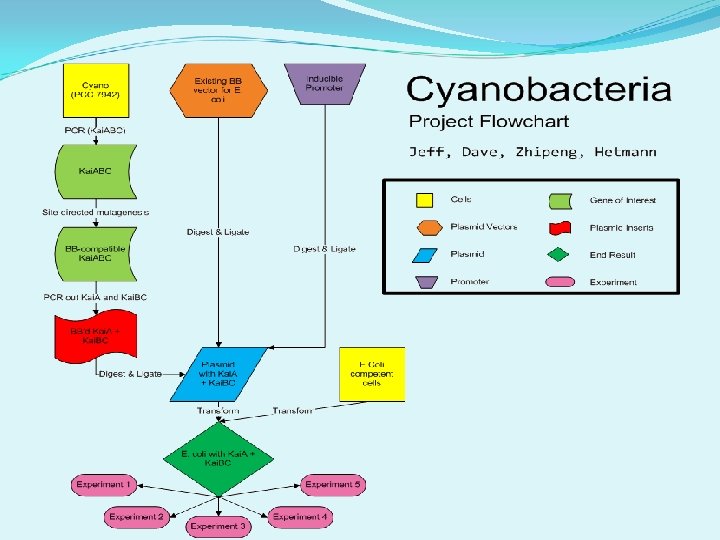
1. Create Kai. A and Kai. BC biobricks. 2. Transform E. coli with Kai Biobricks to reconstitute Kai. C phosphorylation cycle with no reporter attached. 3. Distant: Transform E. coli with Kai Biobricks to reconstitute Kai. C phosphorylation cycle with Biobrick’d luciferase reporter.


1. Using PCR to create and extract the correct constructs. 2. Synchronizing the Kai cycle within one E. coli cell. 3. Pick inducible promoters for Kai. A and Kai. BC. 4. Performing Western blots to detect Kai. C within E. coli.

Grew liquid cultures and plates from the new PCC 7942. Extracted 3 kb Kai. ABC segment using PCR. Began crossover PCR for site-specific mutagenesis. Planned future experiments with E. coli. Decided to eliminate the branch of our project dealing with cyanobacteria. • Designed primers for sequencing Kai. ABC extract. • • •

Goal: Verify that our Kai. A and Kai. BC Biobricks can be expressed in E. coli. Procedure: 1. Tag Kai. A/BC (e. g. with a fluorescent subunit). 2. Insert the tagged Kai. A/BC into E. coli. 3. Induce promoters for both Kai. A and Kai. BC. 4. Measure the E. coli fluorescence. We should see some fluorescence, indicating that our promoters are working and the Kai genes are being expressed. Kai. A 1. Kai. BC

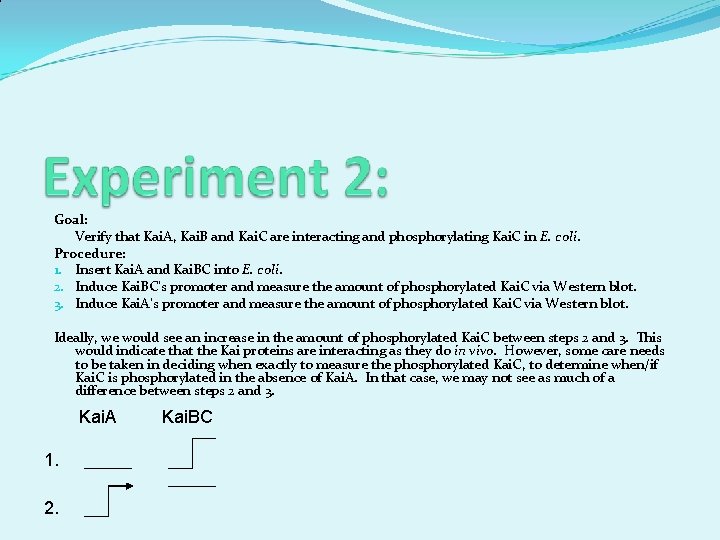
Goal: Verify that Kai. A, Kai. B and Kai. C are interacting and phosphorylating Kai. C in E. coli. Procedure: 1. Insert Kai. A and Kai. BC into E. coli. 2. Induce Kai. BC’s promoter and measure the amount of phosphorylated Kai. C via Western blot. 3. Induce Kai. A’s promoter and measure the amount of phosphorylated Kai. C via Western blot. Ideally, we would see an increase in the amount of phosphorylated Kai. C between steps 2 and 3. This would indicate that the Kai proteins are interacting as they do in vivo. However, some care needs to be taken in deciding when exactly to measure the phosphorylated Kai. C, to determine when/if Kai. C is phosphorylated in the absence of Kai. A. In that case, we may not see as much of a difference between steps 2 and 3. Kai. A 1. 2. Kai. BC

Goal: To measure the oscillation of the Kai. C phosphorylation cycle given pulsed Kai. A and Kai. BC production. Procedure: 1. Insert Kai. A and Kai. BC into E. coli. 2. Induce the promoter for Kai. A and Kai. BC at the same time. 3. After a short time, turn off the promoters. 4. Measure the amount of phosphorylated Kai. C at regular intervals (e. g. every hour) via Western blot. We hope to initially see strong oscillation, with the amplitude decreasing over time. Since no Kai. A/B/C should be produced after the initial pulse, we expect that the intracellular concentrations of the Kai proteins would decrease as the E. coli divide. Kai. A 1. Kai. BC

Goal: To measure the oscillation of the Kai. C phosphorylation cycle given constant Kai. A and pulsed Kai. BC production. Procedure: 1. Insert Kai. A and Kai. BC into E. coli. 2. Induce the promoters for Kai. A and Kai. BC. 3. After a short time, turn off the Kai. BC promoter, leaving the Kai. A promoter induced. 4. Measure the amount of phosphorylated Kai. C at regular intervals. We expect that, since Kai. A is produced at a constant (non-oscillating) rate in cyanobacteria, its constant production in E. coli should not interfere with Kai. C’s phosphorylation cycle. The constant production of Kai. A may even strengthen the oscillation, since Kai. A won’t degrade or dilute away. Kai. A 1. Kai. BC

Goal: To measure the oscillation of the Kai. C phosphorylation cycle, given constant Kai. A and Kai. BC production. Procedure: 1. Insert Kai. A and Kai. BC into E. coli. 2. Induce the promoters for Kai. A and Kai. BC. 3. Measure the amount of phosphorylated Kai. C at regular intervals. We do not know how constant Kai. BC transcription will interfere with the oscillation of Kai. C (in cyanobacteria, Kai. BC transcription oscillates on a circadian rhythm). This experiment should help us figure it out. Kai. A 1. Kai. BC

• Extract Kai. A and Kai. BC coding sequences from Kai. ABC region. • Select inducible promoters for experiments. • Create experiment constructs. • Learn Western blot protocol. • Perform experiments and measure results. • Design new experiments as the need arises.