Water health pollution We are slowly harming our




















- Slides: 24

Water health & pollution Ø We are slowly harming our planet to the point where organisms are dying at a very alarming rate. Ø In addition our drinking water has become greatly affected.

Point source pollution Ø Point sources of pollution occur when harmful substances are emitted from a specific point. Ø Can be traced to a specific point. Ø Ex- The Exxon Valdez oil spill. Ø Factory drainage pipe.

Non point source pollution Ø Non point source pollution is not from a specific source. Ø Instead it comes from a variety of sources. Ø Cannot be traced back to 1 specific spot. Ø Much more difficult to control. Ø Accounts for a majority of the contaminants in streams and lakes. Ex- Farm runoff(Herbicide, pesticide, fertilizer) l Road runoff (oil, gasoline, antifreeze,


Pollution Many causes of pollution including sewage and fertilizers contain nutrients such as nitrates and phosphates. Ø In excess levels, nutrients over stimulate the growth of aquatic plants and algae. Ø Excessive growth uses up dissolved oxygen as algae decompose, and block light to deeper waters. Ø Ø Ø Video- Restricted waters 6 min

Ø Chlorine Ø Chemical added to water to kill bacteria and microorganisms. Ø Swimming pools, hot tubs Ø City drinking water

Dissolved Oxygen Ø The higher the D. O. the healthier the water & supports more diverse aquatic life. Ø 6 ppm= very healthy Ø <3 ppm= stressful to organisms Ø <2 ppm= No fish

Ø Dissolved Oxygen Ø Concentration of Oxygen Ø Most important indicator of the overall health of a river. Ø Without O 2, organisms cannot live.

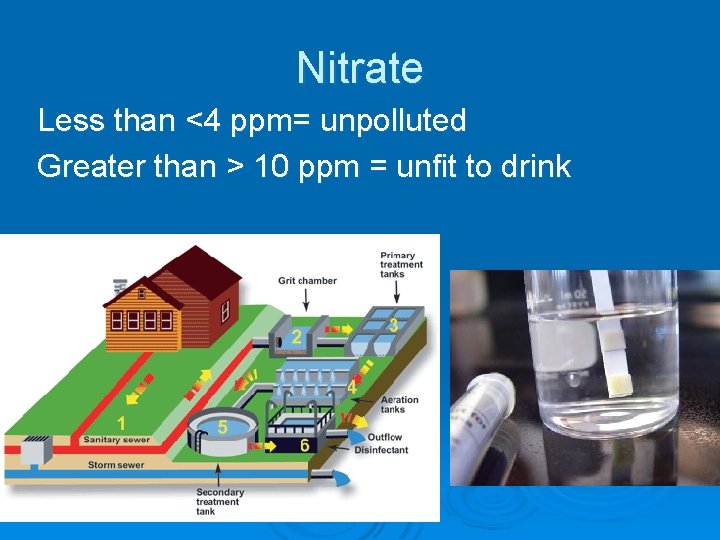
Ø What is nitrate? Ø Nitrate is a form of nitrogen that typically comes from fertilizers, decaying organic matter, wastewater from commercial operations, animal manure and human sewage. Ø It is an odorless, colorless, and tasteless compound. Can cause health problems if it enters drinking water.

Effects Too much nitrate causes an overgrowth of algae & other plants which foul the water. Source Farming & sewage treatment plants Industrial waste


Nitrate Less than <4 ppm= unpolluted Greater than > 10 ppm = unfit to drink



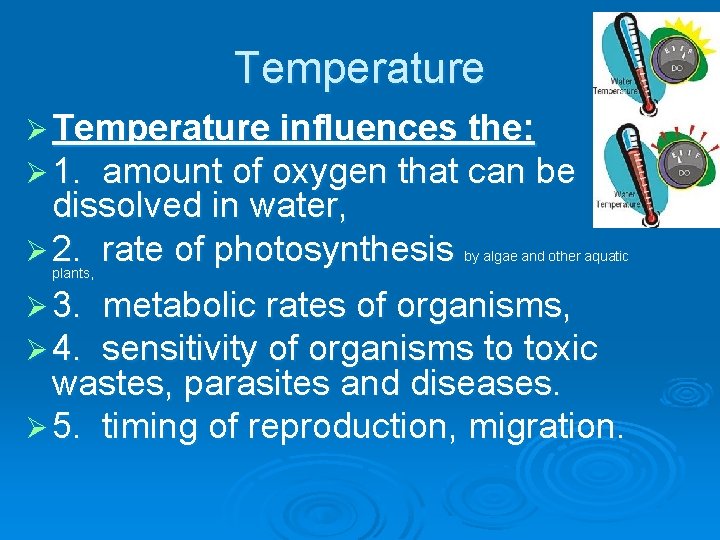
Ø Temperature Ø One of the most important water indicators. Ø Temperature affects water chemistry and the functions of aquatic organisms.

Temperature Ø Temperature influences the: Ø 1. amount of oxygen that can be dissolved in water, Ø 2. rate of photosynthesis by algae and other aquatic plants, Ø 3. Ø 4. metabolic rates of organisms, sensitivity of organisms to toxic wastes, parasites and diseases. Ø 5. timing of reproduction, migration.

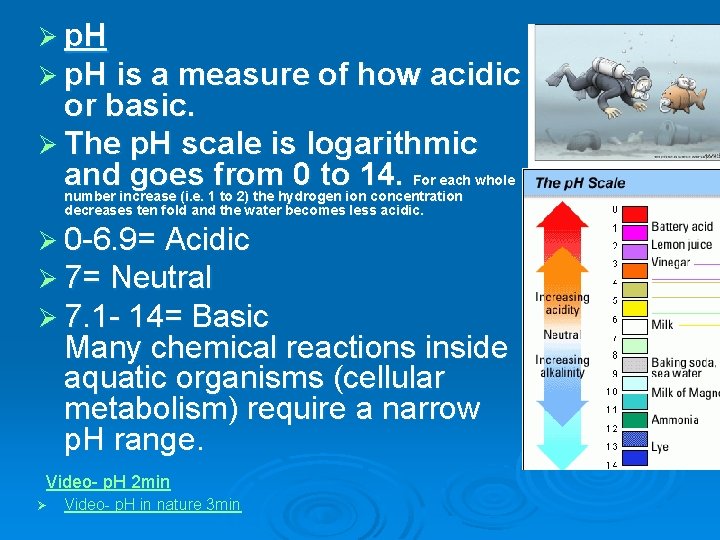
Ø p. H is a measure of how acidic or basic. Ø The p. H scale is logarithmic and goes from 0 to 14. For each whole number increase (i. e. 1 to 2) the hydrogen ion concentration decreases ten fold and the water becomes less acidic. Ø 0 -6. 9= Acidic Ø 7= Neutral Ø 7. 1 - 14= Basic Many chemical reactions inside aquatic organisms (cellular metabolism) require a narrow p. H range. Video- p. H 2 min Ø Video- p. H in nature 3 min

Ø Turbidity is a measure of the amount of suspended particles in the water. Ø Algae, suspended sediment, and organic matter particles can cloud the water. Ø Particles diffuse sunlight and absorb heat. Ø Increasing temperature and reduce light available for algal photosynthesis.

Ø Secci disk is used to measure turbidity.

Phosphate Ø Phosphates come from fertilizer, waste water from dish & laundry soap and from industry waste. Ø Causes overgrowth of algae. Ø Algae decomposition uses up O 2. Ø Fish & other organisms die.

Phosphate

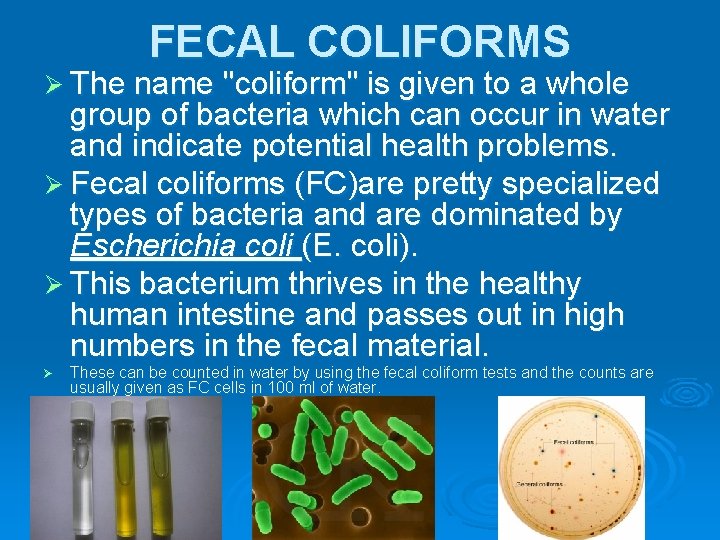
FECAL COLIFORMS Ø The name "coliform" is given to a whole group of bacteria which can occur in water and indicate potential health problems. Ø Fecal coliforms (FC)are pretty specialized types of bacteria and are dominated by Escherichia coli (E. coli). Ø This bacterium thrives in the healthy human intestine and passes out in high numbers in the fecal material. Ø These can be counted in water by using the fecal coliform tests and the counts are usually given as FC cells in 100 ml of water.

Escherichia coli is common in human intestine, usually not harmful. Ø (Some strains can cause infections. ) Ø FC is a widely accepted indicator of the potential pollution of water with fecal material. Ø Suggests possibility of infectious microorganisms in the water. Ø These organisms include viruses, other bacteria, protozoa and a variety of worms. Ø Ø Video Bacteria 3 min

 Mikael ferm
Mikael ferm Water and water and water water
Water and water and water water Groundwater pollution
Groundwater pollution Section 3 water pollution
Section 3 water pollution Type of reefs
Type of reefs Negative effect of water pollution
Negative effect of water pollution What are the terrible twelve water pollution
What are the terrible twelve water pollution Solutions to water pollution
Solutions to water pollution Water pollution impact on ecosystem
Water pollution impact on ecosystem Effects of land pollution on human health
Effects of land pollution on human health Measures of noise pollution
Measures of noise pollution Land water and air pollution
Land water and air pollution Methods of water pollution
Methods of water pollution Effects of water pollution
Effects of water pollution Ddt water pollution
Ddt water pollution What are 5 effects of water pollution?
What are 5 effects of water pollution? Objectives of water pollution
Objectives of water pollution Way to reduce water pollution
Way to reduce water pollution Conclusion of water pollution
Conclusion of water pollution Types of water pollution
Types of water pollution Water pollution and unequal distribution illustration
Water pollution and unequal distribution illustration Summary of water pollution
Summary of water pollution Ap environmental science chapter 11
Ap environmental science chapter 11 Environmental science chapter 11
Environmental science chapter 11 Sources of thermal pollution
Sources of thermal pollution