WARMUP WarmUp 121014 12914 1 Get Howatwould you












- Slides: 14

WARM-UP Warm-Up 12/10/14 12/9/14 -1) Get Howatwould you name the following least two different color utensils. compounds? 2) Draw the Lewis dot structures of the NCl 3 following N 2 Oelements: BCl 3 3 H, C, N, O, Cl N H NI SO 2 4 3 3) For each of the elements above, 3 write down how many pairs of electrons there Na 3 P Mg. O Ca. F 2 are and how many unpaired electrons there are.

Objectives Review atoms and the periodic table Agenda Review Warm-Up Part A of Study Guide Kahoot Poll

Monday Tuesday Wednesday Thursday Review Part Review A Part B ? ? Friday ? ? Finals: Periods 1 Periods 2 & Periods 3 Periods 6 &4 5 &7 &8 - EVERYONE must show up for finals. - Absent = Incomplete = no grade

WARM-UP Warm-Up 12/10/14 12/9/14 -1) Get Howatwould you name the following least two different color utensils. compounds? 2) Draw the Lewis dot structures of the NCl 3 following N 2 Oelements: BCl 3 3 H, C, N, O, Cl N H NI SO 2 4 3 3) For each of the elements above, 3 write down how many pairs of electrons there Na 3 P Mg. O Ca. F 2 are and how many unpaired electrons there are.

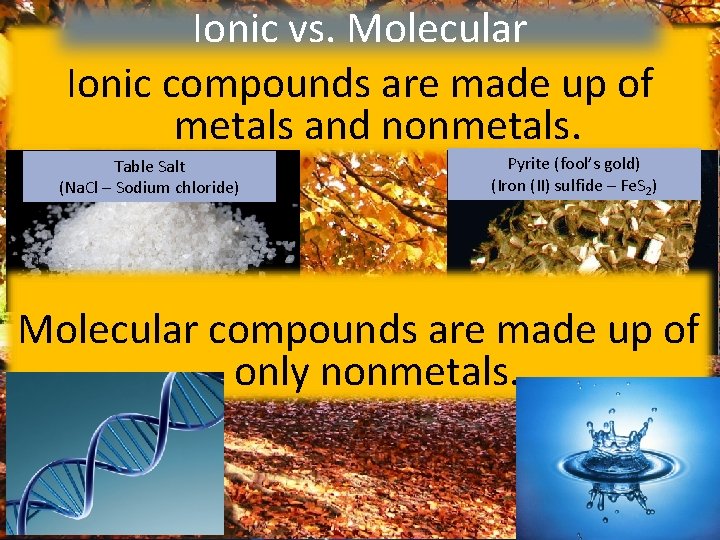
Ionic Warm-Up vs. Molecular 12/9/14 Ionic compounds are made up of 1) Get at least two different color utensils. metals and nonmetals. 2) Draw ofgold)the Pyrite (fool’s Table Saltthe Lewis dot structures (Iron (II) sulfide – Fe. S ) (Na. Cl – Sodium chloride) following elements: H, C, N, O, Cl 3) For each of the elements above, write Molecular compounds are made up of down how many pairs of electrons there only nonmetals. are and how many unpaired electrons there are. 2

Rules for Naming Covalent Compounds • First, need to recognize that it is a covalent compound. • How do we do that? ? Covalent compounds should only contain nonmetals!

Rules for Naming Covalent Compounds Similar to ionic compound naming • Name the first element that comes first • The second element drops the ending and adds –ide BUT! • The names need prefixes.

Rules for Naming Covalent Compounds • CO vs. CO 2 CO would be carbon monoxide CO 2 would be carbon dioxide • Both elements need prefixes EXCEPT when there is only one of the first element.

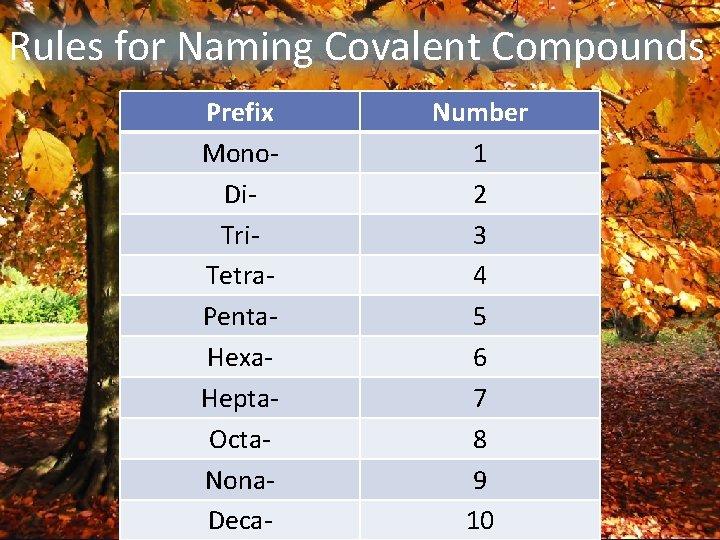
Rules for Naming Covalent Compounds Prefix Mono. Di. Tri. Tetra. Penta. Hexa. Hepta. Octa. Nona. Deca- Number 1 2 3 4 5 6 7 8 9 10

Debrief DIHYDROGEN MONOXIDE (DHMO) -Can be found all around the world. -The U. S. uses about 346, 000 million gallons every day.

Debrief DIHYDROGEN MONOXIDE (DHMO) Pros -Is found in the human body. - It can be used as a coolant. -Plant and animal life need it to live. -It is a necessary ingredient in many foods and almost all beverages.

Debrief DIHYDROGEN MONOXIDE (DHMO) Cons -The major component of acid rain. -Contributes to the Greenhouse Effect. -May cause severe burns. -May cause electrical failures and decreased effectiveness of automobile brakes.

Debrief - On a separate sheet of paper, answer: - Based on what you learned today about naming molecular compounds and dihydrogen monoxide, do you think it should be banned? Why or why not? - DON’T FORGET YOUR NAME!

Homework Part B of Final Review Study Guide