Valence Electrons Valence n n n Valence electrons








- Slides: 8

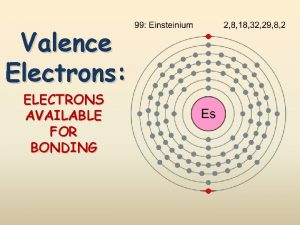
Valence Electrons

Valence n n n Valence electrons are the electrons that are involved in chemical bonding. They have the highest amount of energy and are found in the outermost electron shell, furthest from the nucleus. The electron dot diagram shows the chemical symbol surrounded by the valence electrons. dot diagram for neon dot diagram for phosphorous

Trading Electrons n n n Chemical change involves trading valence electrons. Atoms are stable when they have 8 valence electrons. To become stable, atoms may either lose, gain or share electrons to form a chemical bond – a force of attraction between two atoms as a result of a rearrangement of electrons. Not balanced. Needs ___ electrons? Balanced with 8.

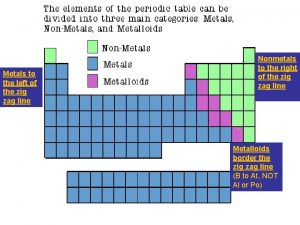
What’s your Valence? n n Hydrogen is grouped with the metals even though it is a nonmetal because it has _____ valence electron. group 1 (alkali metals) ¨ 1 n group 2 (alkaline earth metals) ¨ 2 n valence electrons group 13 ¨ 3 n valence electrons group 14 ¨ 4 valence electrons

1. What are valence electrons and what do we use to represent them? 2. What role do valence electrons play in the formation of compounds from elements? 3. How do the properties of elements change as you move across the periodic table from left to right, and what does this have to do with valence electrons? 4. Draw an electron dot diagram and atomic model for: a) nitrogen, b) magnesium c) helium d)sulfur 5. How many valence electrons do the noble gases have, and why are they so stable?

Valence continued n n n group 15 ¨ 5 valence electrons group 16 ¨ 6 valence electrons group 17 (the halogens) ¨ 7 valence electrons group 18 (noble gases) ¨ 8 and are stable and unreactive as a result groups 3 -12 (transition metals) ¨ 2, 3, or 4 valence electrons

More Practice Draw electron dot diagrams for: magnesium, chlorine, potassium, krypton, carbon n

Practice! n Draw electron dot diagrams for the following elements: ¨ Calcium, oxygen, silicon, argon