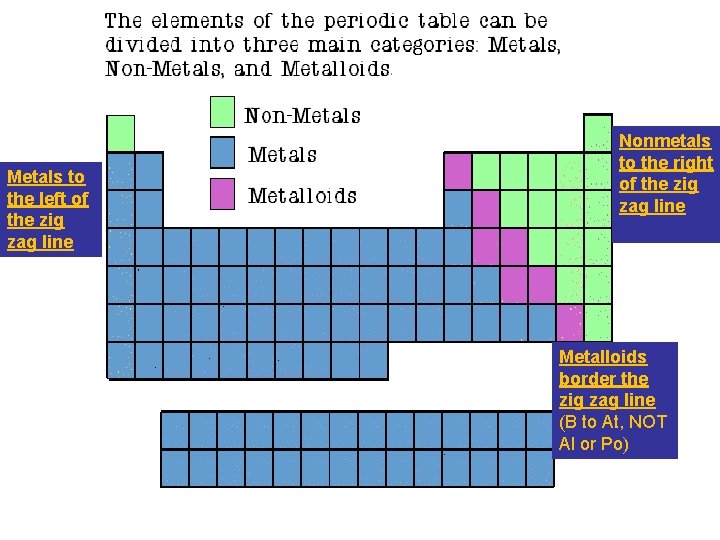
Metals to the left of the zig zag




















- Slides: 53

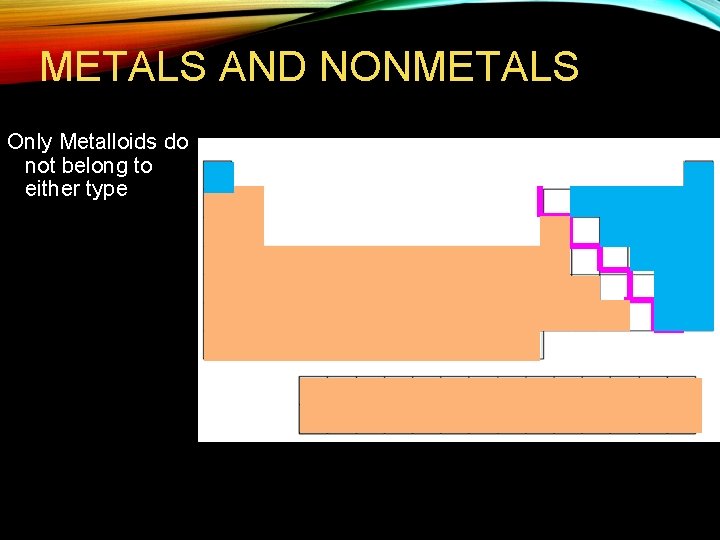
Metals to the left of the zig zag line Nonmetals to the right of the zig zag line Metalloids border the zig zag line (B to At, NOT Al or Po)

PERIODIC TABLE NOTES The Elements and the Table Notes in packet and color code your periodic table

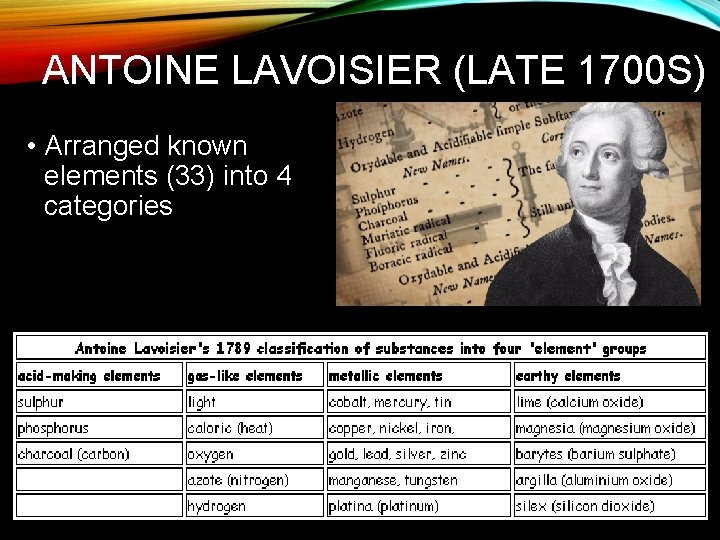
ANTOINE LAVOISIER (LATE 1700 S) • Arranged known elements (33) into 4 categories

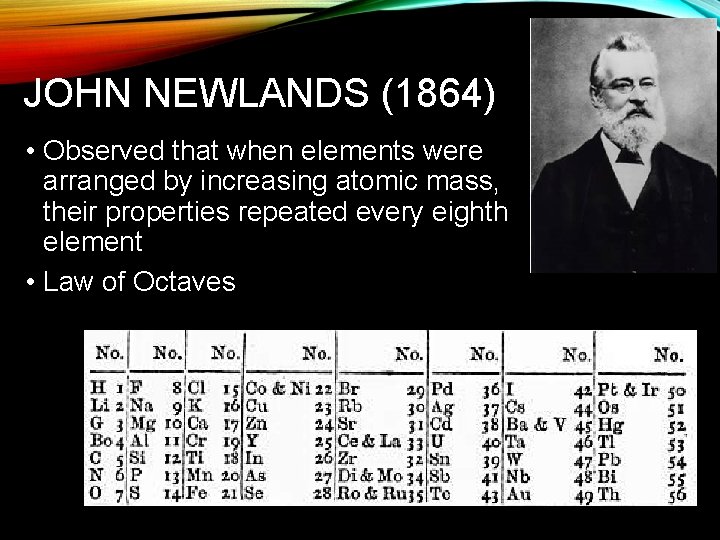
JOHN NEWLANDS (1864) • Observed that when elements were arranged by increasing atomic mass, their properties repeated every eighth element • Law of Octaves

DMITRI MENDELEEV In 1869 he published a table of the elements organized by increasing atomic mass.

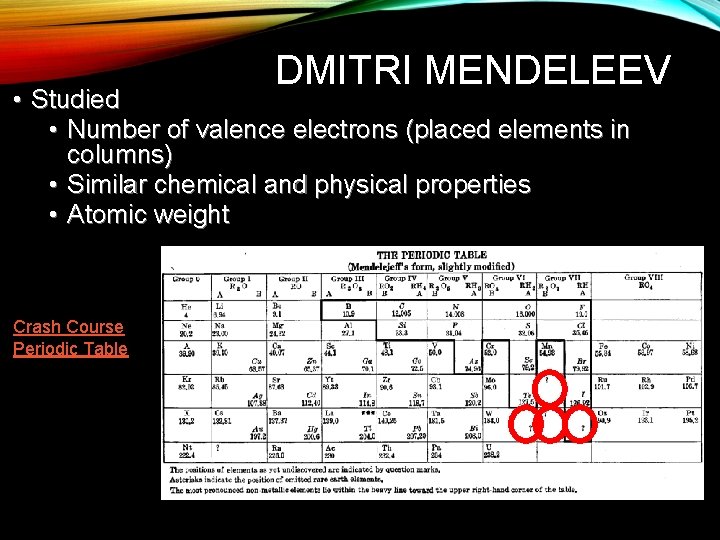
DMITRI MENDELEEV • Studied • Number of valence electrons (placed elements in columns) • Similar chemical and physical properties • Atomic weight Crash Course Periodic Table

HENRY MOSELEY (1913) Mos Pros!!! • With discovery of new elements and accurate atomic masses, some elements were not in the correct placement • Arranged periodic table by increasing atomic number • Periodic repetition of chemical and physical properties that resulted is periodic law

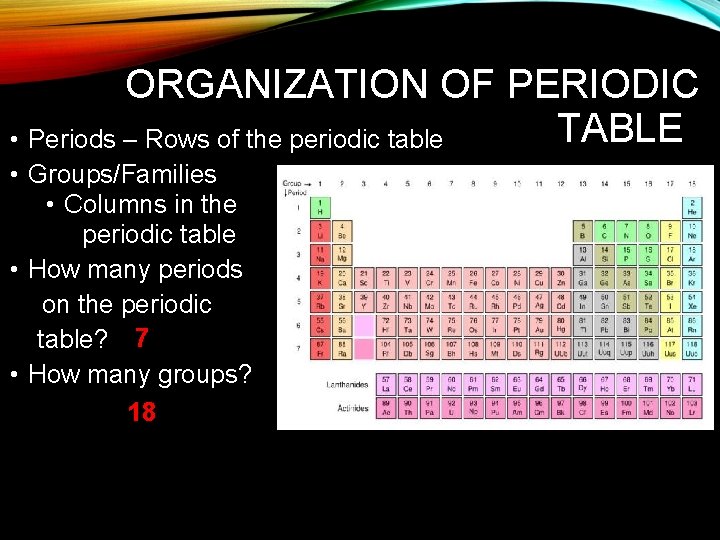
ORGANIZATION OF PERIODIC TABLE • Periods – Rows of the periodic table • Groups/Families • Columns in the periodic table • How many periods on the periodic table? 7 • How many groups? 18

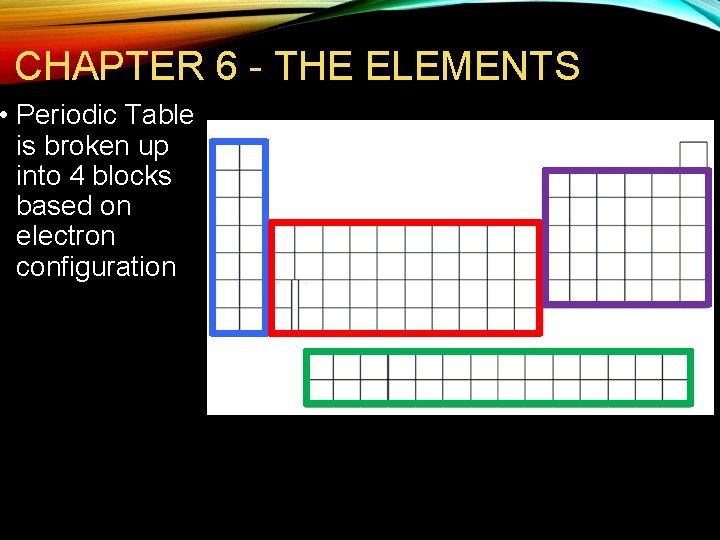
CHAPTER 6 - THE ELEMENTS • Periodic Table is broken up into 4 blocks based on electron configuration

CHAPTER 6 - THE ELEMENTS • 92 natural elements (all after U man-made, also Tc and Pm are man-made) • Groups 1, 2 and 13 -18 = representative elements (periodic law applies to them) • Chemical and physical properties recur periodically when arranged by increasing atomic number

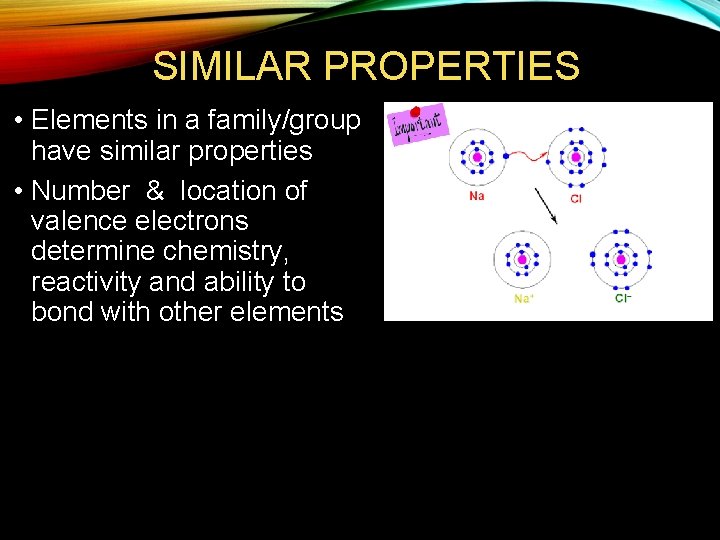
SIMILAR PROPERTIES • Elements in a family/group have similar properties • Number & location of valence electrons determine chemistry, reactivity and ability to bond with other elements

METALS AND NONMETALS Only Metalloids do not belong to either type

METALS VS. NONMETALS Metals tend to LOSE e • metal reactivity increases ↓ a group, and ← a period • Weaker attraction with nucleus makes it easier to lose Nonmetals tend to GAIN e • nonmetal reactivity increases ↑ a group and → a period • Stronger attraction with nucleus makes it easier to gain

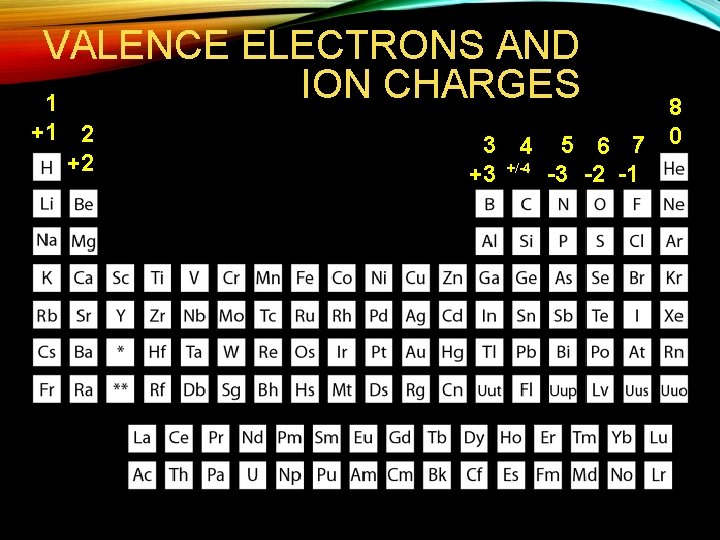
VALENCE ELECTRONS AND ION CHARGES 1 +1 2 +2 8 3 4 5 6 7 0 +3 +/-4 -3 -2 -1

Metals, Nonmetals and metalloids Metals Shiny when smooth and clean Solid at room temp. Good conductors of heat and electricity Malleable Ductile n Nonmetals – Generally gases or brittle, dull-looking solids – Poor conductors of heat and electricity • Metalloids – Have physical and chemical properties of both metals and nonmetals!

STOP!!!! • Complete the table on the bottom of page 4!!!

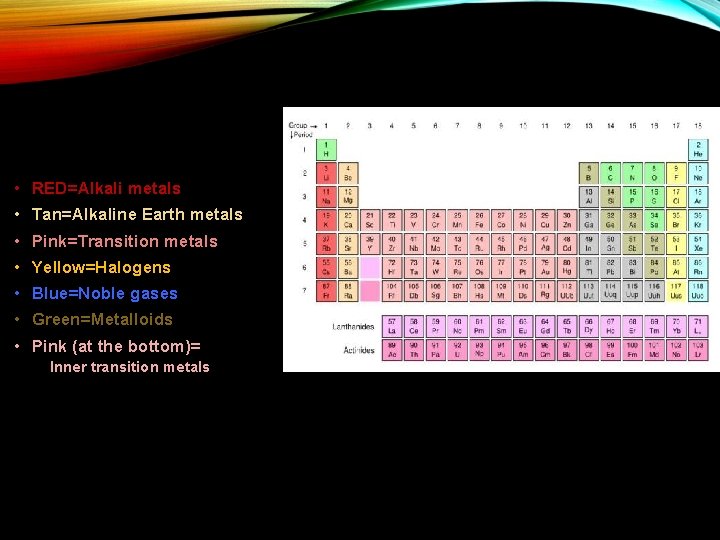
• RED=Alkali metals • Tan=Alkaline Earth metals • Pink=Transition metals • Yellow=Halogens • Blue=Noble gases • Green=Metalloids • Pink (at the bottom)= Inner transition metals

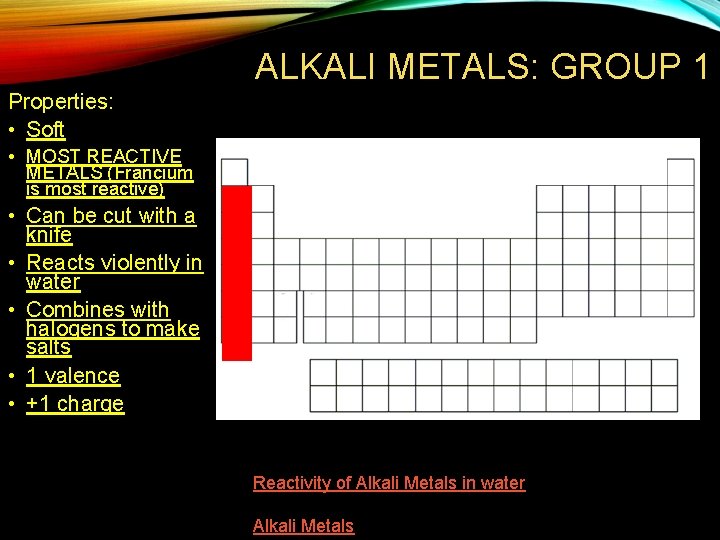
ALKALI METALS: GROUP 1 Properties: • Soft • MOST REACTIVE METALS (Francium is most reactive) • Can be cut with a knife • Reacts violently in water • Combines with halogens to make salts • 1 valence • +1 charge Reactivity of Alkali Metals in water Alkali Metals

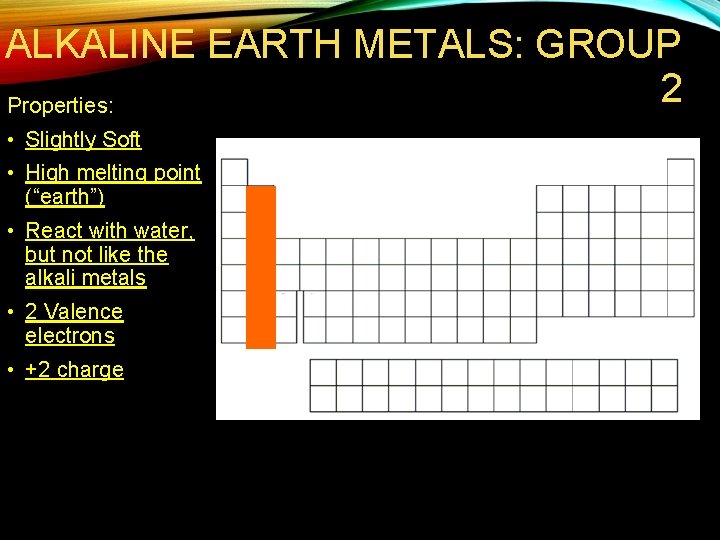
ALKALINE EARTH METALS: GROUP 2 Properties: • Slightly Soft • High melting point (“earth”) • React with water, but not like the alkali metals • 2 Valence electrons • +2 charge

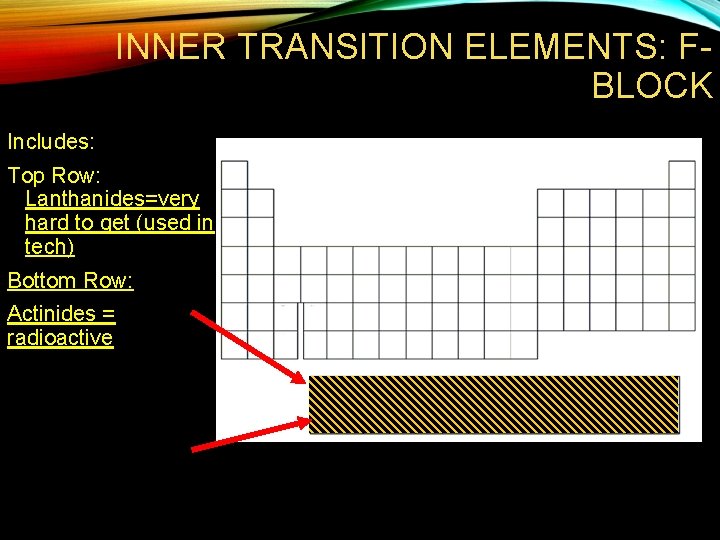
INNER TRANSITION ELEMENTS: FBLOCK Includes: Top Row: Lanthanides=very hard to get (used in tech) Bottom Row: Actinides = radioactive

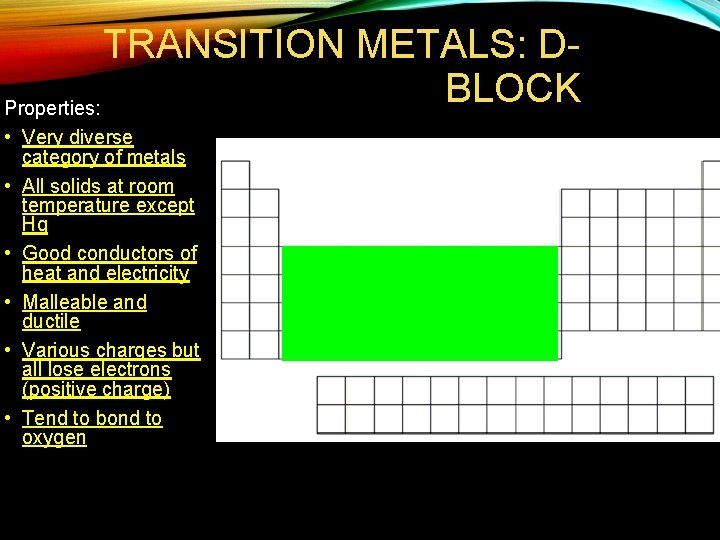
TRANSITION METALS: DBLOCK Properties: • Very diverse category of metals • All solids at room temperature except Hg • Good conductors of heat and electricity • Malleable and ductile • Various charges but all lose electrons (positive charge) • Tend to bond to oxygen

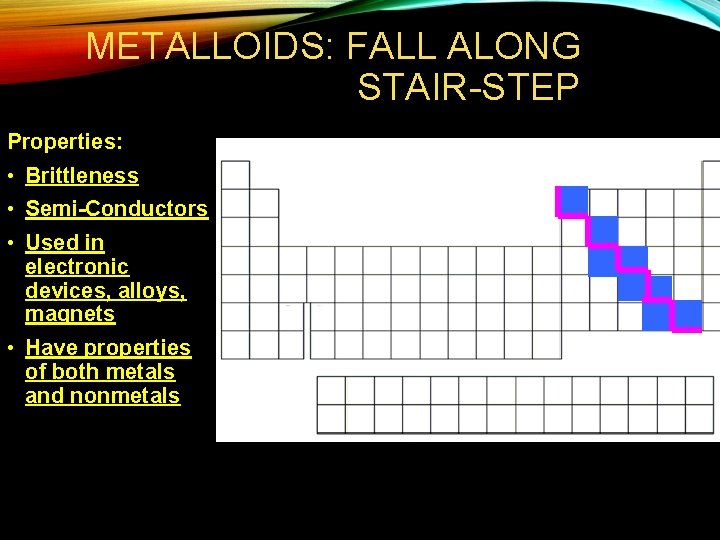
METALLOIDS: FALL ALONG STAIR-STEP Properties: • Brittleness • Semi-Conductors • Used in electronic devices, alloys, magnets • Have properties of both metals and nonmetals

HALOGENS: GROUP 17 Latin for: salt maker Properties: • Form strong acids • React w/ metals to form salts • HIGHLY REACTIVE NONMETALS (Fluorine is the most reactive) • 7 valence electrons • -1 charge Halogens video

NOBLE GASES: GROUP 18 Properties: • MOSTLY Nonreactive • Has FULL outer shell which makes them stable (octet) • 8 valence • No charge Noble Gases

STOP!!! • Complete page 7 in your packet!!!

PERIODIC TRENDS Notes start on page 11

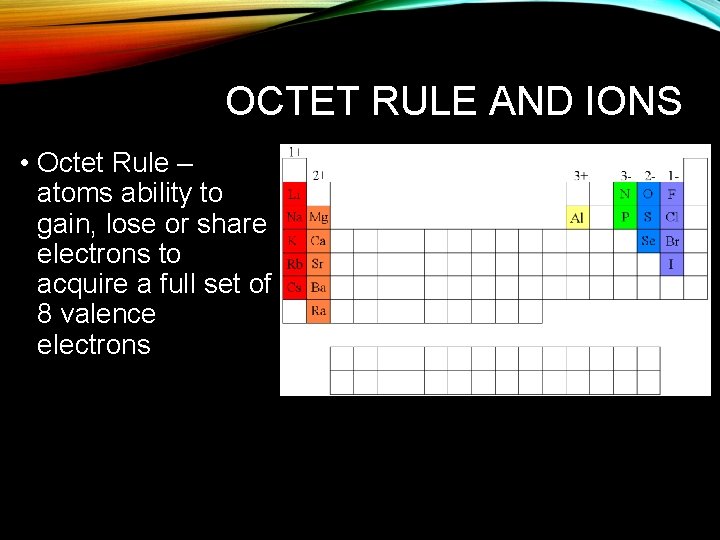
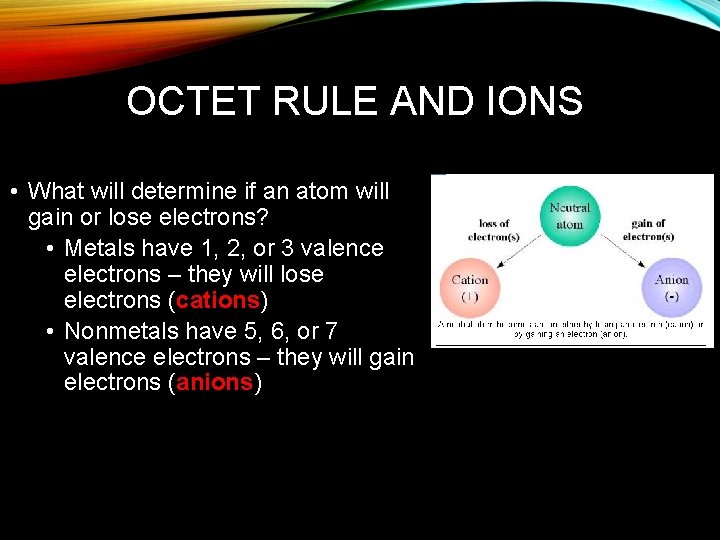
OCTET RULE AND IONS • Octet Rule – atoms ability to gain, lose or share electrons to acquire a full set of 8 valence electrons

OCTET RULE AND IONS • What will determine if an atom will gain or lose electrons? • Metals have 1, 2, or 3 valence electrons – they will lose electrons (cations) • Nonmetals have 5, 6, or 7 valence electrons – they will gain electrons (anions)

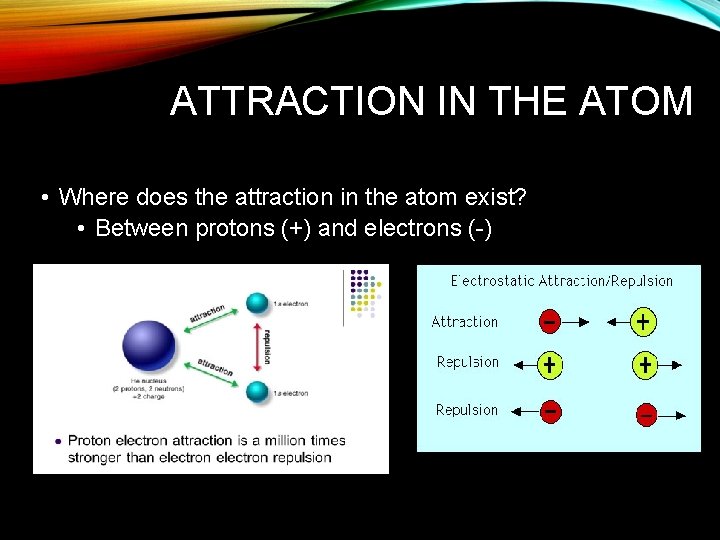
ATTRACTION IN THE ATOM • Where does the attraction in the atom exist? • Between protons (+) and electrons (-)

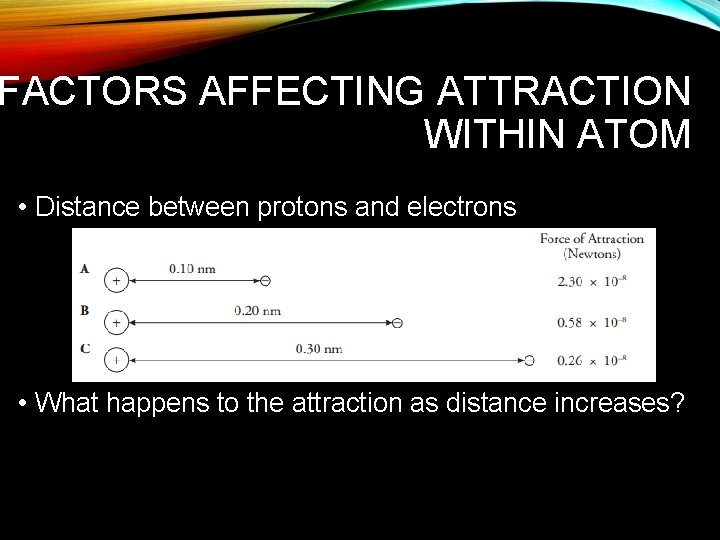
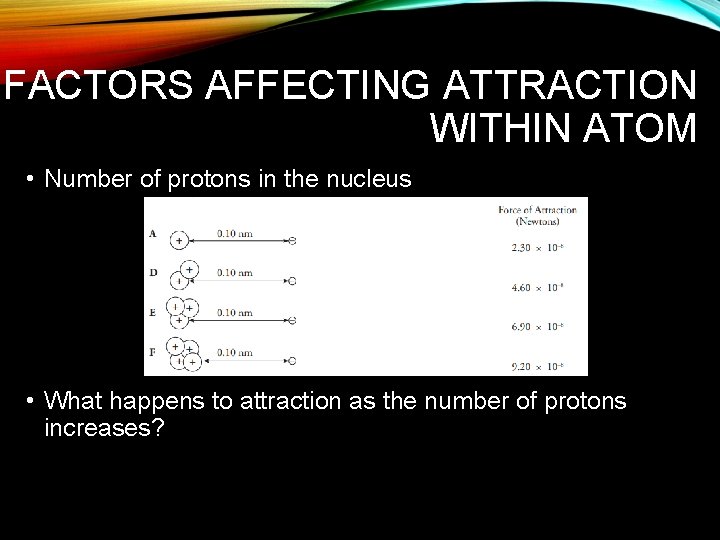
FACTORS AFFECTING ATTRACTION WITHIN ATOM • Distance between protons and electrons • What happens to the attraction as distance increases?

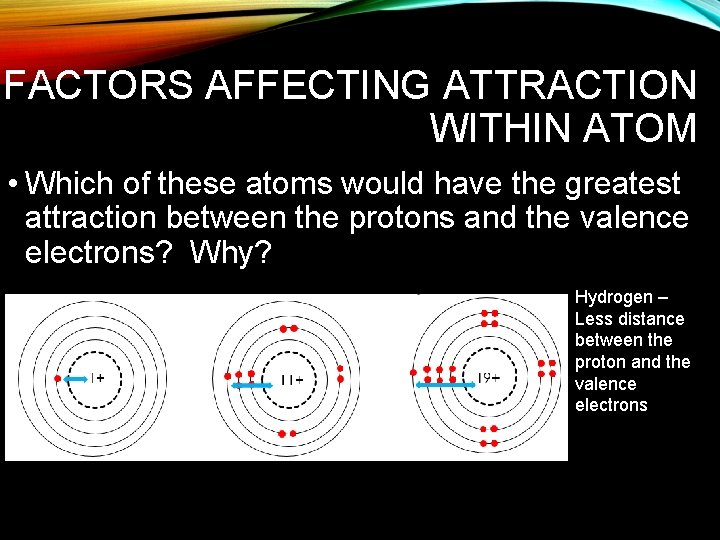
FACTORS AFFECTING ATTRACTION WITHIN ATOM • Which of these atoms would have the greatest attraction between the protons and the valence electrons? Why? Hydrogen – Less distance between the proton and the valence electrons

FACTORS AFFECTING ATTRACTION WITHIN ATOM • Number of protons in the nucleus • What happens to attraction as the number of protons increases?

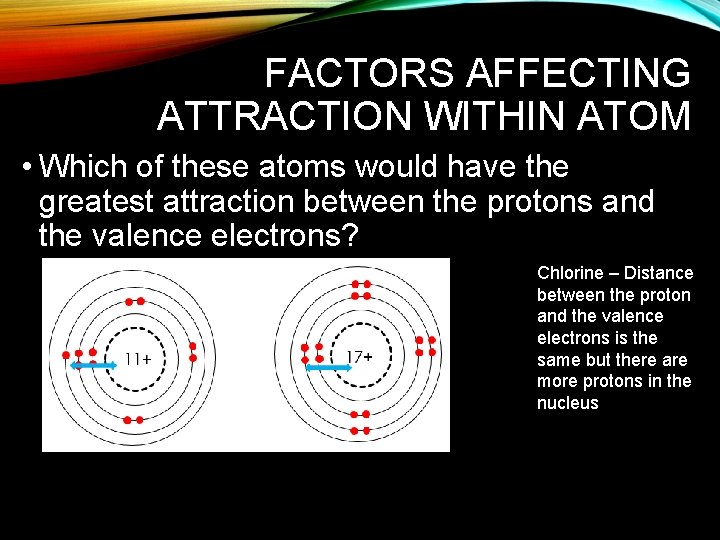
FACTORS AFFECTING ATTRACTION WITHIN ATOM • Which of these atoms would have the greatest attraction between the protons and the valence electrons? Chlorine – Distance between the proton and the valence electrons is the same but there are more protons in the nucleus

10/24 • Pick up the straw activity paper • Take out your notes • We discuss quiz results and then go through a couple of trends together. You will do a straw activity BEFORE we talk about 4 MAIN TRENDS. • TOMORROW: you will be given the entire day to work on the project IF TODAY GOES WELL. If you do not bring your project to work on, you will have an alternate assignment that will be due at the end of the class. • HW: page 11 -14 goes with the notes. I will check these on tomorrow. • FYI: Test on 11/1 (1/2 summative) AND Project DUE TUESDAY 10/29 (1/2 summative)

TRENDS ON TEST: TAKE NOTES ON PAGE 12 -14 • 1. Effective Nuclear Charge • 2. Shielding Effect • 3. Reactivity • 4. Electronegativity • 5. Ionization Energy • 6. Atomic Radius • 7. Ionic Radius (4 -7 are the main trends)

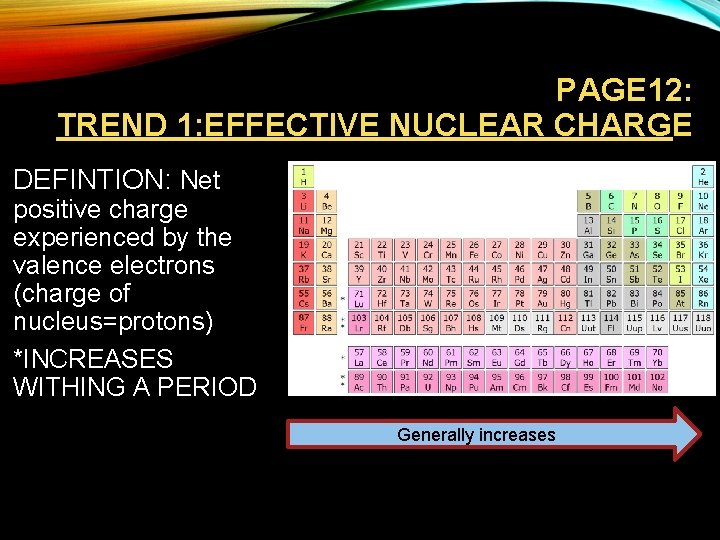
PAGE 12: TREND 1: EFFECTIVE NUCLEAR CHARGE • DEFINTION: Net positive charge experienced by the valence electrons (charge of nucleus=protons) • *INCREASES WITHING A PERIOD Generally increases

PAGE 12: TREND 2: SHIELDING EFFECT • Shielding of outer electrons by inner CORE electrons • Shields the outer electrons from the positive pull of the nucleus

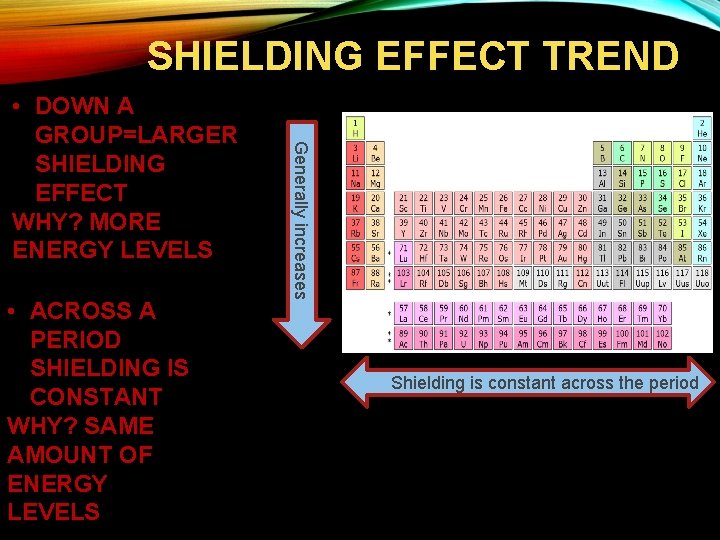
SHIELDING EFFECT TREND • ACROSS A PERIOD SHIELDING IS CONSTANT WHY? SAME AMOUNT OF ENERGY LEVELS Generally increases • DOWN A GROUP=LARGER SHIELDING EFFECT WHY? MORE ENERGY LEVELS Shielding is constant across the period

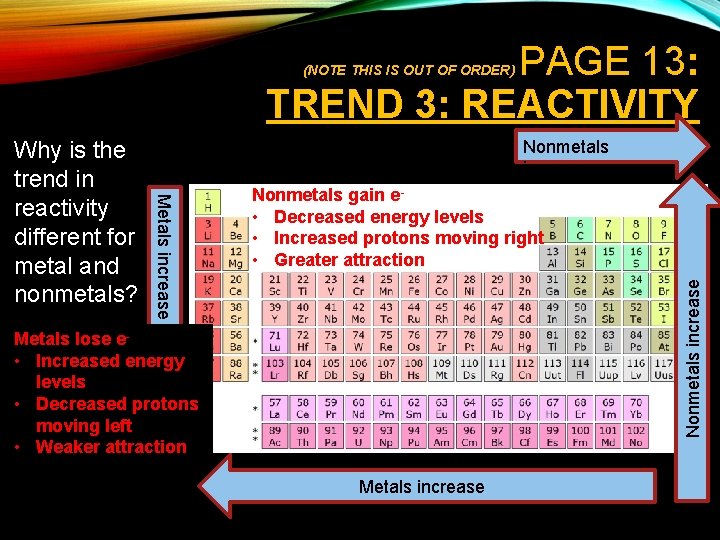
PAGE 13: TREND 3: REACTIVITY (NOTE THIS IS OUT OF ORDER) Nonmetals increase Nonmetals gain e • Decreased energy levels • Increased protons moving right • Greater attraction Nonmetals increase Metals increase Why is the trend in reactivity different for metal and nonmetals? Metals lose e • Increased energy levels • Decreased protons moving left • Weaker attraction Metals increase


TRENDS ON TEST: TAKE NOTES ON PAGE 12 -14 • 1. Effective Nuclear Charge • 2. Shielding Effect • 3. Reactivity • 4. Electronegativity • 5. Ionization Energy • 6. Atomic Radius • 7. Ionic Radius (4 -7 are the main trends)

STRAWS LAB • Each group of 3 will be given one of the four main trends to make a model of. • You will answer the following questions on a blank piece of paper BEFORE you can make your model: • 1. What is your trend? • 2. What is the overall trend (using arrows)? • 3. Define your trend. • 4. Calculate the values OF EACH ELEMENT IN ROWS 2 -5 that you are ask for in PROCEDURE #2 on the lab. PUT THESE IN A TABLE THAT MATCHES THE ONE GIVEN TO YOU. • You must bring me the answers to the above questions, so I can sign off on them. AFTER YOU GET MY SIGNATURE, PERFORM PROCEDURE #3. When you are finished, place your model on the lab station that corresponds to your trend AND START ANSWERING THE QUESTIONS ON THE “periodic trends straw activity” you picked up at the beginning of class. THIS IS A GRADE, MAKE SURE YOU ARE ANSWERING CORRECTLY.

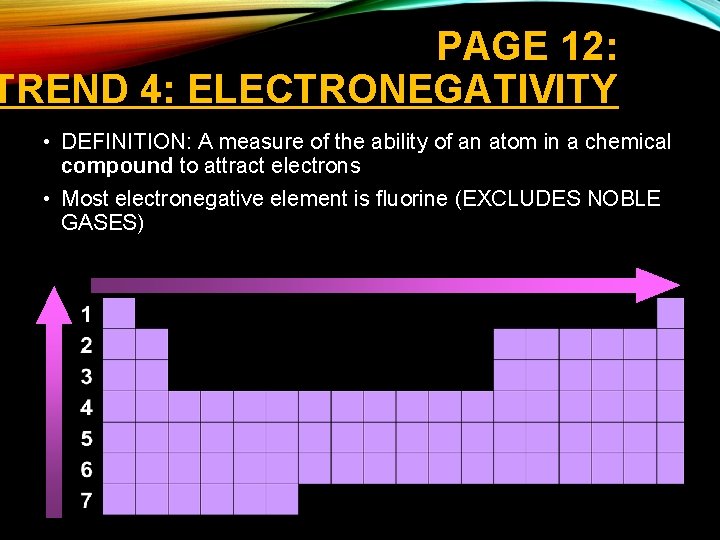
PAGE 12: TREND 4: ELECTRONEGATIVITY • DEFINITION: A measure of the ability of an atom in a chemical compound to attract electrons • Most electronegative element is fluorine (EXCLUDES NOBLE GASES)

ELECTRONEGATIVITY TREND • Why would we see higher electro-negativities in the nonmetals than we do in the metals? • Why does EN increase moving up the group?

LET’S PRACTICE • Between the elements As, Sn and Sb, which is the most electronegative? As • Between the elements Sr, Ba and Ra, which is the most electronegative? Sr

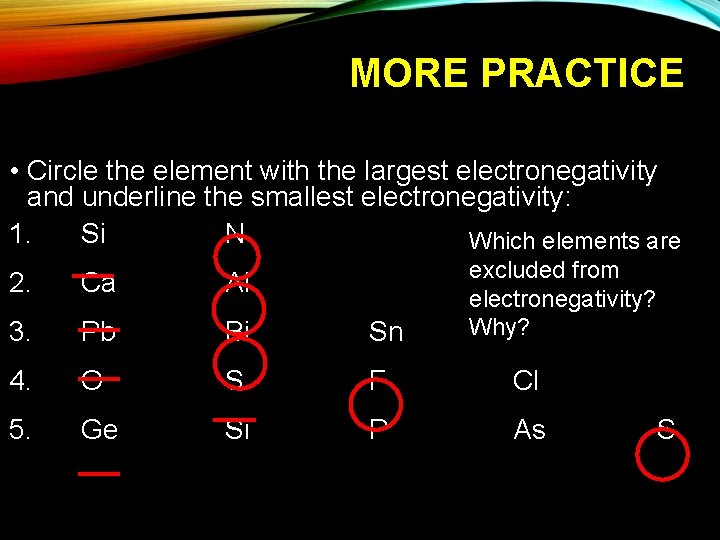
MORE PRACTICE • Circle the element with the largest electronegativity and underline the smallest electronegativity: 1. Si N Which elements are excluded from electronegativity? Why? 2. Ca Al 3. Pb Bi Sn 4. O S F Cl 5. Ge Si P As S

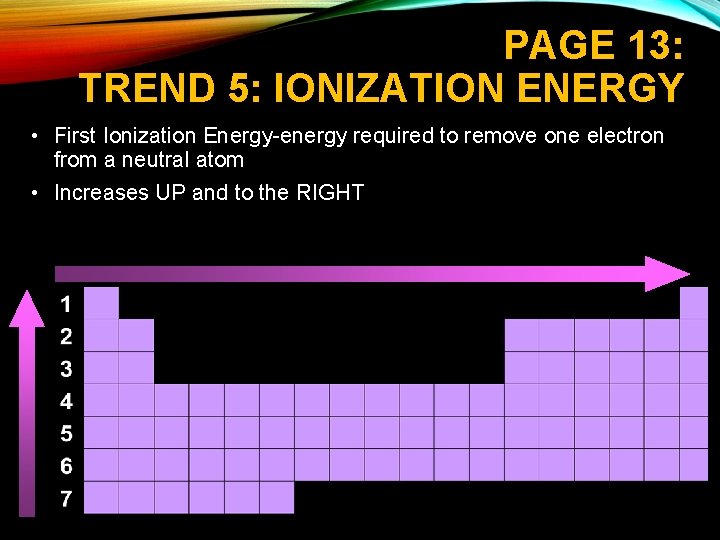
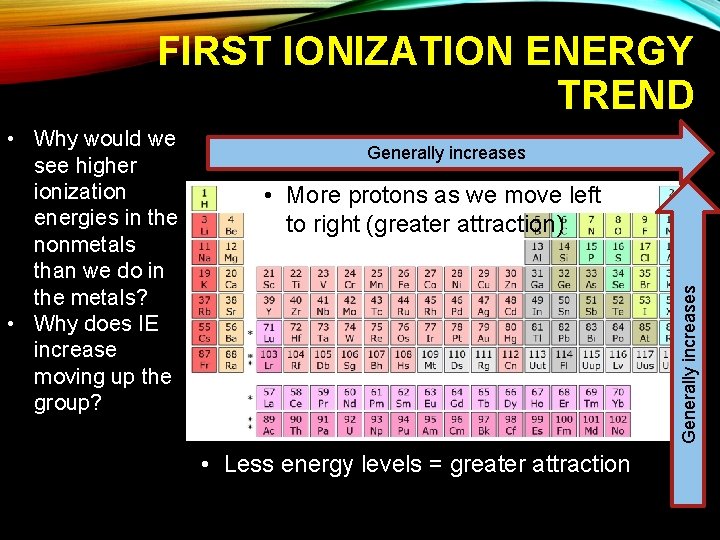
PAGE 13: TREND 5: IONIZATION ENERGY • First Ionization Energy-energy required to remove one electron from a neutral atom • Increases UP and to the RIGHT

FIRST IONIZATION ENERGY TREND Generally increases • More protons as we move left to right (greater attraction) Generally increases • Why would we see higher ionization energies in the nonmetals than we do in the metals? • Why does IE increase moving up the group? • Less energy levels = greater attraction

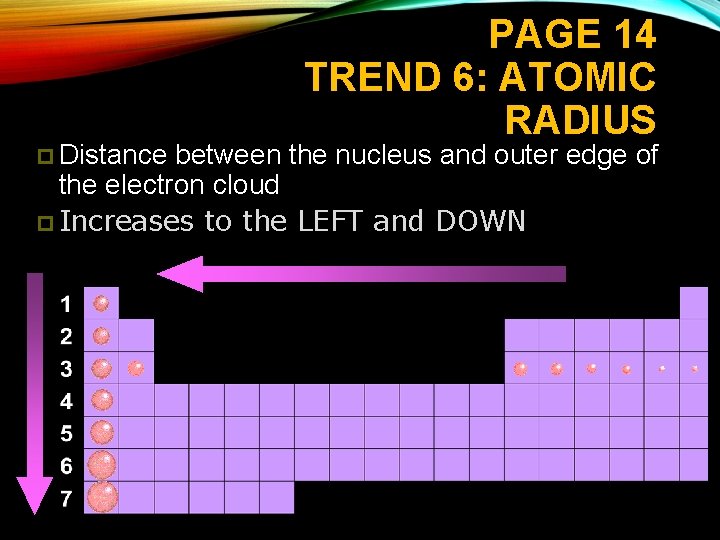
PAGE 14 TREND 6: ATOMIC RADIUS p Distance between the nucleus and outer edge of the electron cloud p Increases to the LEFT and DOWN

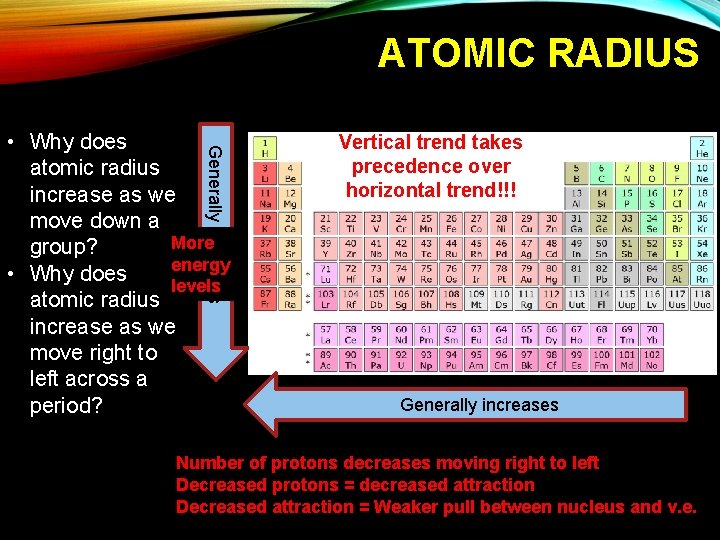
ATOMIC RADIUS Generally increases • Why does atomic radius increase as we move down a More group? energy • Why does levels atomic radius increase as we move right to left across a period? Vertical trend takes precedence over horizontal trend!!! Generally increases Number of protons decreases moving right to left Decreased protons = decreased attraction Decreased attraction = Weaker pull between nucleus and v. e.

LET’S PRACTICE • Between the elements Po, Te, I, which one has the largest atomic radius? • • Po Between the elements Sc, Rb and Cr, which one has the smallest atomic radius? • Cr

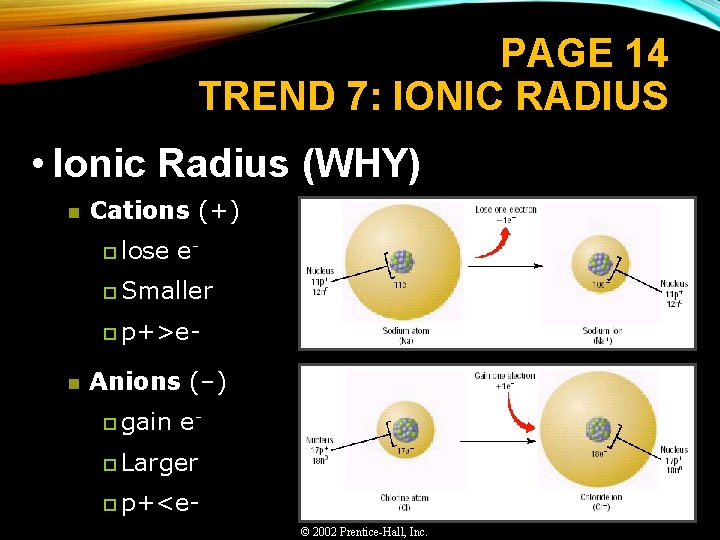
PAGE 14 TREND 7: IONIC RADIUS • Ionic Radius (WHY) n Cations (+) p lose e- p Smaller p p+>e- n Anions (–) p gain e- p Larger p p+<e© 2002 Prentice-Hall, Inc.

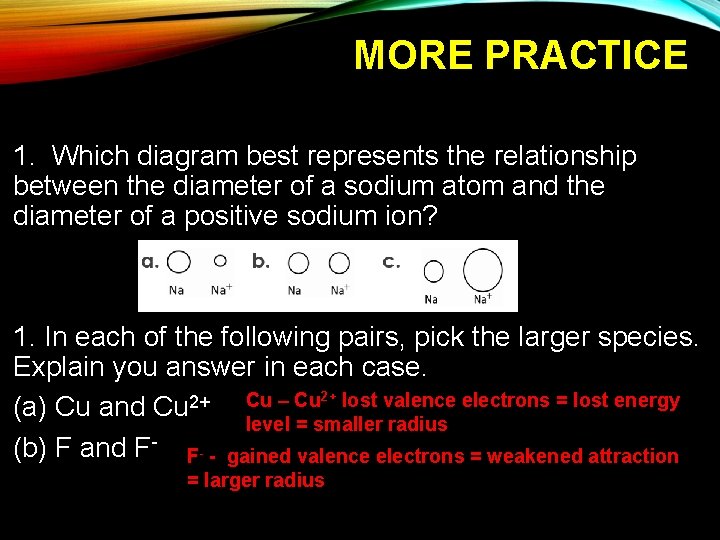
MORE PRACTICE 1. Which diagram best represents the relationship between the diameter of a sodium atom and the diameter of a positive sodium ion? 1. In each of the following pairs, pick the larger species. Explain you answer in each case. 2+ lost valence electrons = lost energy Cu – Cu 2+ (a) Cu and Cu level = smaller radius (b) F and F- F- - gained valence electrons = weakened attraction = larger radius
 Nch soldadura
Nch soldadura Soldadura en v
Soldadura en v Il principio di funzionamento del trasformatore
Il principio di funzionamento del trasformatore Zig zag kanał
Zig zag kanał Técnica de arrastre limpieza
Técnica de arrastre limpieza A rivet is a cylindrical bar
A rivet is a cylindrical bar Indice horaire transformateur
Indice horaire transformateur Transformateur zig zag
Transformateur zig zag Lateral barrier hop
Lateral barrier hop Examples of non metals
Examples of non metals Density of metalloids
Density of metalloids Grade 7 natural science term 3 worksheets
Grade 7 natural science term 3 worksheets Non metals periodic table
Non metals periodic table Natural science grade 7 matter and materials
Natural science grade 7 matter and materials Examples of alloy metals
Examples of alloy metals Hoe zag nederland er vroeger uit
Hoe zag nederland er vroeger uit Zorgafstemmingsgesprek
Zorgafstemmingsgesprek For top-down parsing left recursion removal is
For top-down parsing left recursion removal is Go the building and turn left
Go the building and turn left Put your left foot in
Put your left foot in Muscle energy technique si joint
Muscle energy technique si joint Turn around go go go
Turn around go go go Left left right right go go go
Left left right right go go go Downstage and upstage
Downstage and upstage Zig bi
Zig bi Zig iterator
Zig iterator Jovanovo otkrovenje zig zveri
Jovanovo otkrovenje zig zveri Zig heile
Zig heile Zig bi
Zig bi Mrd marketing requirements document
Mrd marketing requirements document Zig memory allocation
Zig memory allocation Hilary hinton ziglar
Hilary hinton ziglar Otkrovenje jovanovo zig zveri
Otkrovenje jovanovo zig zveri Zig interface
Zig interface Zig-bee automation market
Zig-bee automation market Zig tutorial
Zig tutorial Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 So nguyen to
So nguyen to Tia chieu sa te
Tia chieu sa te Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em ưu thế lai là gì
ưu thế lai là gì Sơ đồ cơ thể người
Sơ đồ cơ thể người Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Tư thế ngồi viết
Tư thế ngồi viết Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Công thức tiính động năng
Công thức tiính động năng Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Gấu đi như thế nào
Gấu đi như thế nào Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu









































































