Valence Electrons Group Properties 5 3 Valence Electrons








- Slides: 23

Valence Electrons & Group Properties 5. 3

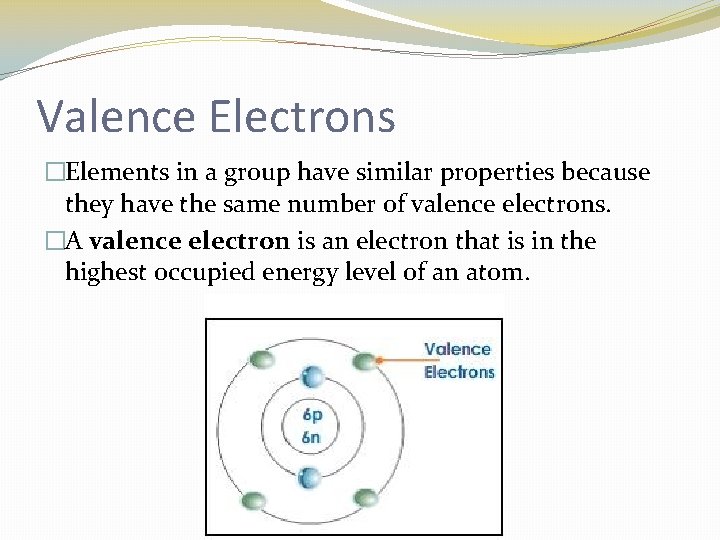
Valence Electrons �Elements in a group have similar properties because they have the same number of valence electrons. �A valence electron is an electron that is in the highest occupied energy level of an atom.

Valence Electrons & Group Number When the A groups in the periodic table are numbered from 1 through 8, the group number matches the number of valence electrons in the electron configuration of an element in that group. • Valence electrons play a key role in chemical reactions. • Properties vary across a period because the number of valence electrons increases from left to right.

Alkali Metals �Group 1 A � 1 Valence Electron �Extremely Reactive and thus found only in nature as compounds

Alkaline Earth Metals �Group 2 A � 2 Valence Electrons �Highly Reactive Oyster shell with pearl Spinach plant Plaster cast

Boron Family �Group 3 A � 3 Valence Electrons �Ex: Aluminum = Most abundant metal in Earth’s Crust �Lab Glass materials

Carbon Family �Group 4 A � 4 Valence Electrons

Nitrogen Family �Group 5 A � 5 Valence Electrons �Elements with a wide range of physical properties �Used a lot in fertilizer

Oxygen Family �Group 6 A � 6 Valence Electrons �Ex: Oxygen is the most abundant element in the Earth’s crust

Halogens �Group 7 A � 7 Valence Electrons �Found as gas, liquid or solid �Despite these differences, chemical properties are the same

Noble Gases �He has 2 valence electrons �All the others have 8 �Colorless, odorless and extremely unreactive = STABLE

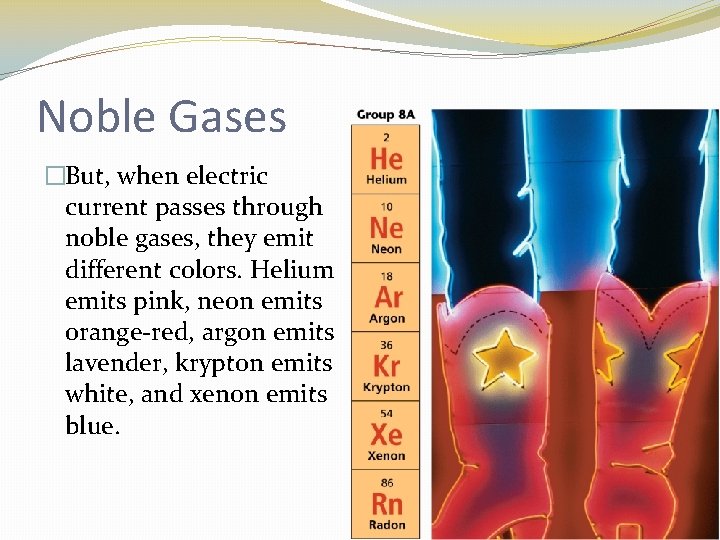
Noble Gases �But, when electric current passes through noble gases, they emit different colors. Helium emits pink, neon emits orange-red, argon emits lavender, krypton emits white, and xenon emits blue.

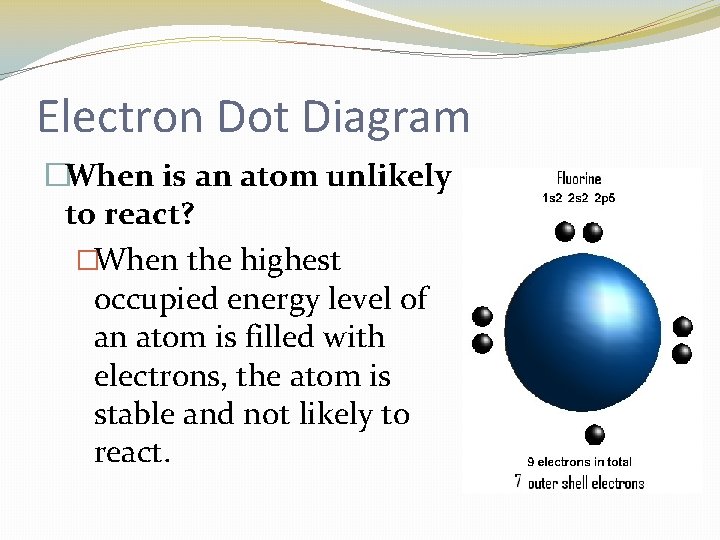
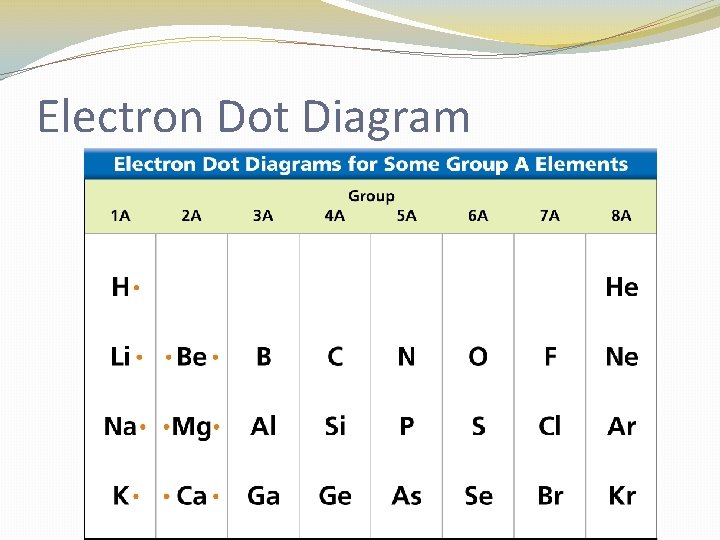
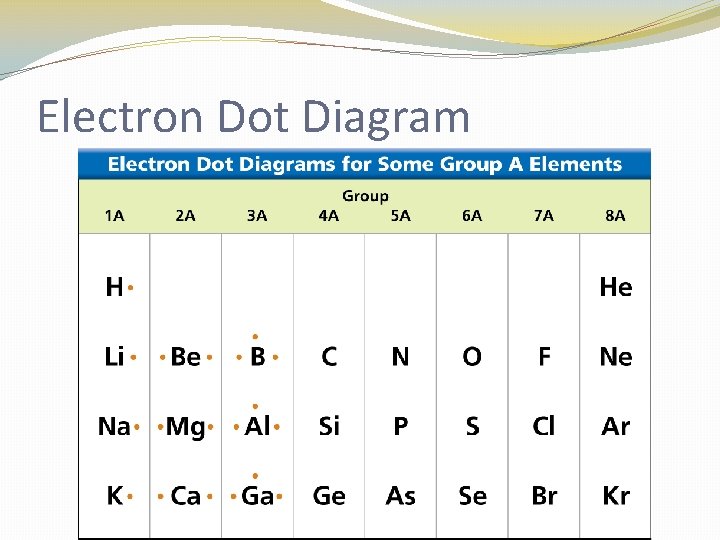
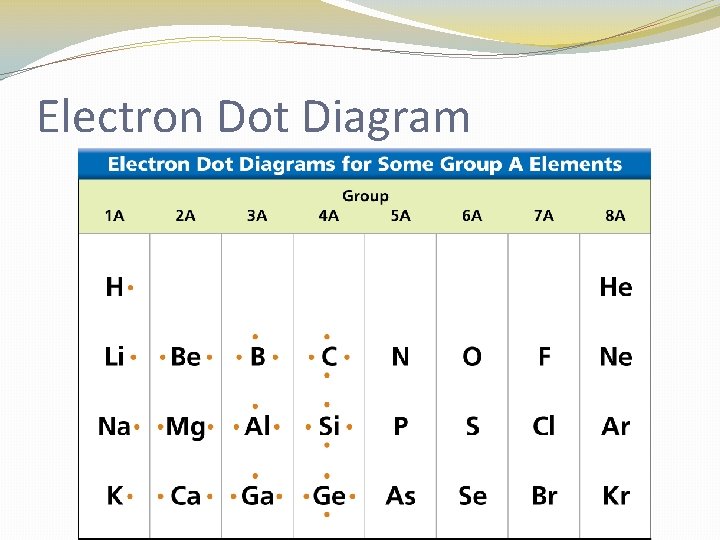
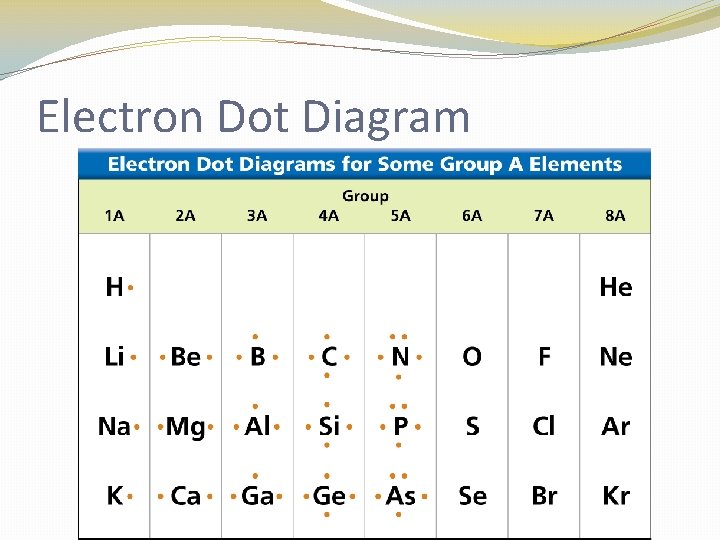
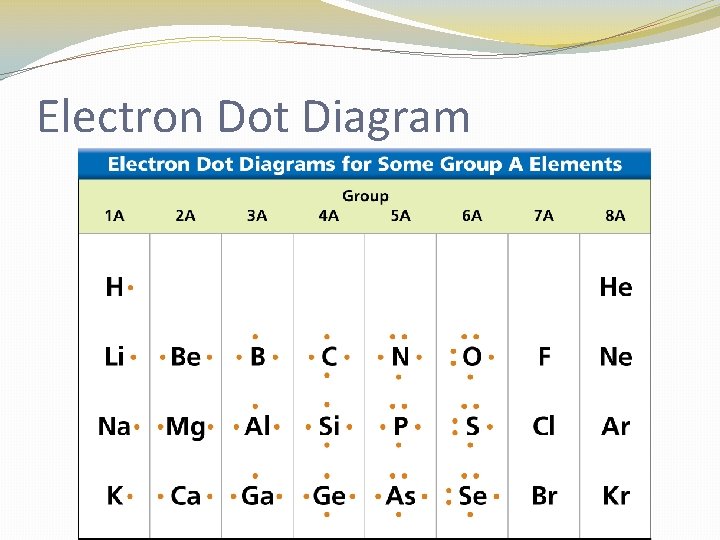
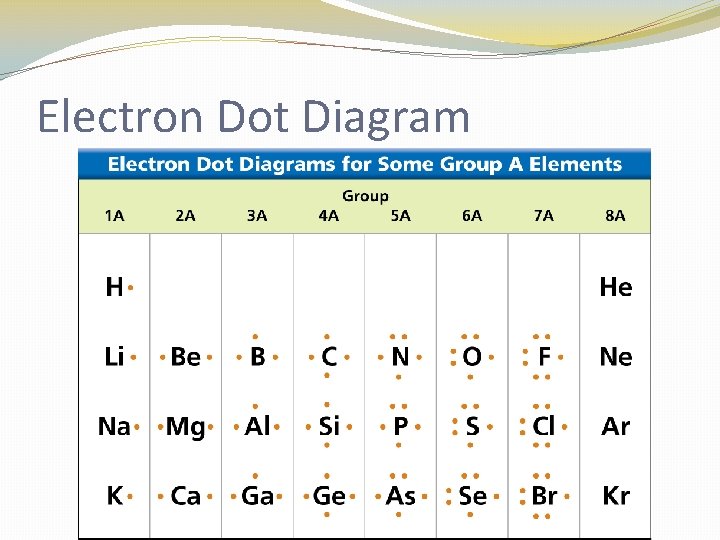
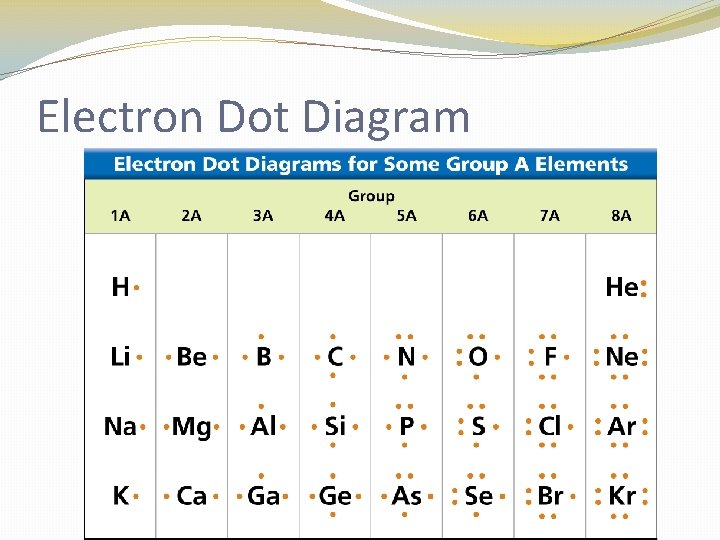
Electron Dot Diagram �When is an atom unlikely to react? �When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react.

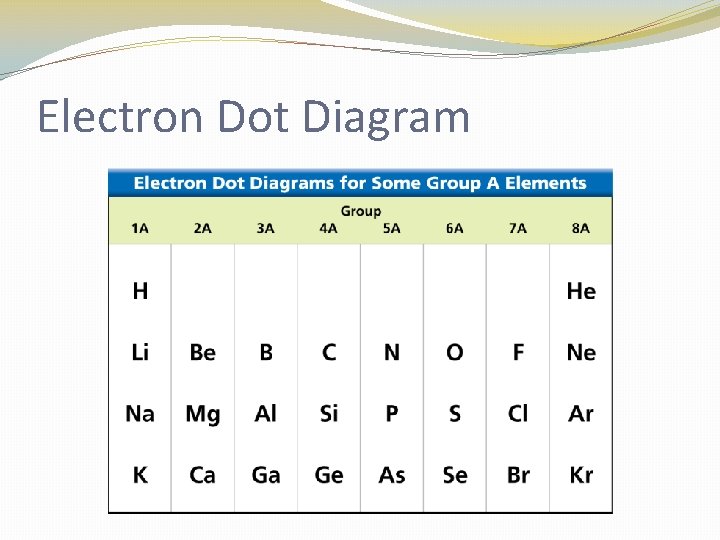
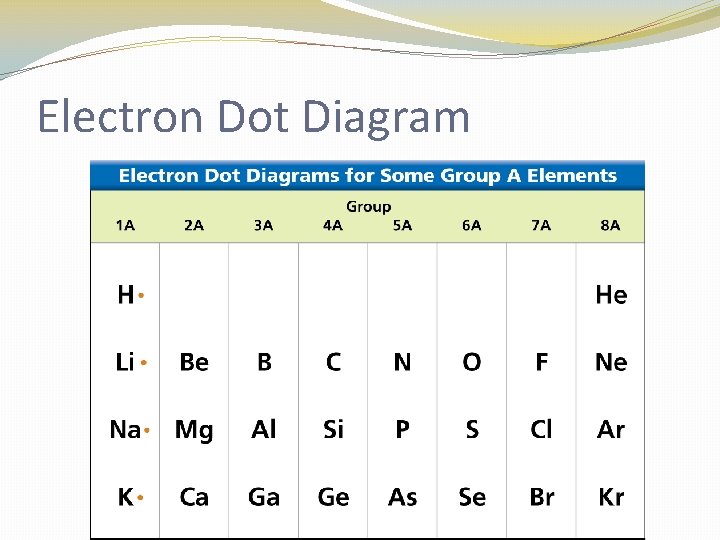
Electron Dot Diagram �The chemical properties of an element depend on the number of valence electrons. An electron dot diagram is a model of an atom in which each dot represents a valence electron. The symbol in the center represents the nucleus and all the other electrons in the atom.

Electron Dot Diagram

Electron Dot Diagram

Electron Dot Diagram

Electron Dot Diagram

Electron Dot Diagram

Electron Dot Diagram

Electron Dot Diagram

Electron Dot Diagram

Electron Dot Diagram