UPDATE OF THE L M GIG MEDITERRANEAN GIG









- Slides: 14

“UPDATE OF THE L- M GIG” MEDITERRANEAN GIG 2 nd MEETING (16 December 2004)

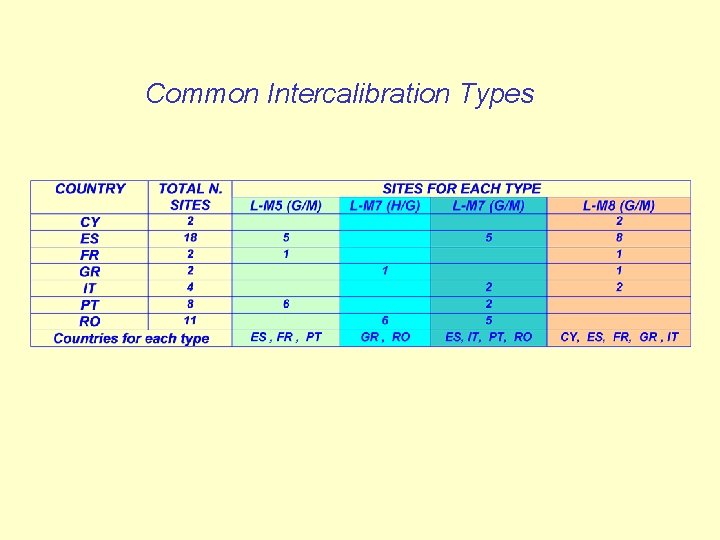
Common Intercalibration Types

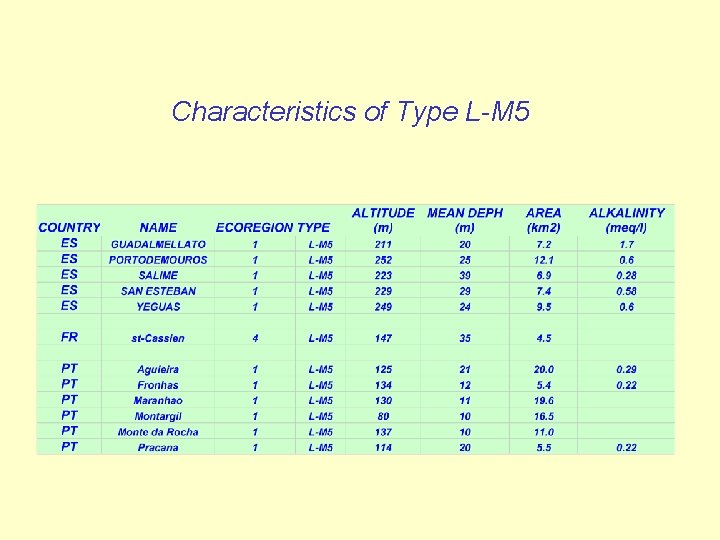
Characteristics of Type L-M 5

Characteristics of Type L-M 7

Characteristics of Type L-M 8

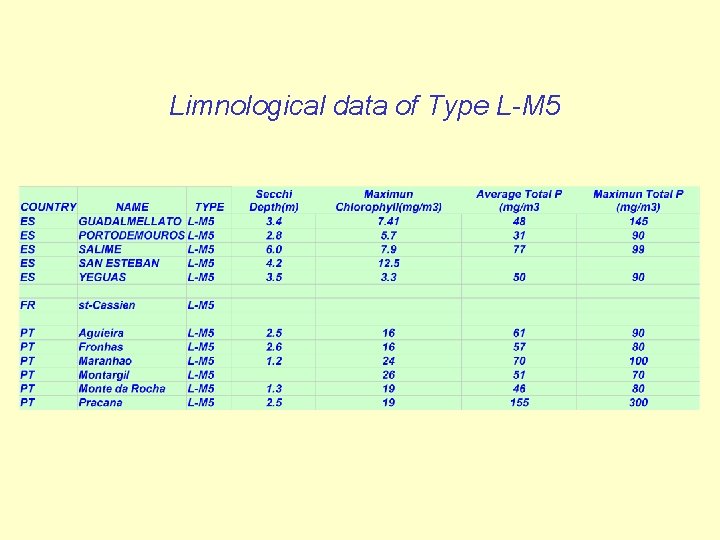
Limnological data of Type L-M 5

Limnological data of Type L-M 7

Limnological data of Type L-M 8

“METHODOLOGICAL ISSUES RELATED WITH CHLOROPHYLL A” MEDITERRANEAN GIG 2 nd MEETING (16 December 2004)

1) Volume of water sample “ 0, 5 to 2 litres is often convenient for sampling and appropriate for the commonly encountered concentration range of 1 -20 mg/m 3 chlorophyll a. In order to obtain extracts of suitable optical density, smaller sample volumes can be used with denser populations. Larger volumes may be desirable for unproductive waters, but may be also inconvenient to obtain or filter” (Vollenweider, 1969). - For Intercalibraton reservoirs could be appropriate to filter between 1 or 2 litres of water. - It is recommended to use opaque bottles.

2) Filtration “Water samples should be filtered through either membrane or glass fibre filters. The pore size of the filters must be sufficiently small to retain all algae of the smallest dimensions. When membrane filters are used, a pore size of 0, 45 mm (Millipore HA) is recommended; when glass fibre filters are employed a pore size of 0. 5 to 0, 7 mm should be used (Whatman GF/F). ( Wetzel and Likens , 1991). Whatman GF/C are also used frequently. Could be appropriate to use Whatman filter GF/F for Intercalibration reservoirs ? “After filtration is completed the filter should be removed and immediately used in the extraction procedure. When extraction cannot be done right away, the filters should be frozen. Samples may be stored for a few days when frozen at – 20 to – 60ºC”. (Wetzel and Likens , 1991).

3) Extraction “Either acetone (80% or 90% aqueous mixture) or Methanol (90% or 100%) can be used as the solvent (Vollenweider, 1969)”. Methanol has the inconvenience of the toxicity and acetone 90 % is used frequently. Other possibilities as mixture 1: 1 of acetone with dimethyl sulphoxide (Shoaf and Liun, 1976), have tested at some Spanish universities obtaining more extracting power of chlorophyll a, although without constant increment (between 1 and 6%). For Intercalibraton reservoirs could be appropriate to use acetona 90% as the solvent because seem the most frequently used at Mediterranean countries. Volumes of extract of 10 or 15 ml are recommended, considering the used volume in the calculation of the concentration of chlorophyll. Extraction period in cool dark conditions (refrigerator) of about 24 hours. After extraction period, the extract is centrifuged and the clear supernatant solution used to fill a spectrophotometer cell.

4) Determination of chlorophyll a: -Spectrophotometric determination: The chlorophylls a, b and c, absorbs ligth maximally at specific wavelengths when dissolved in organic solvents. -Fluorometric determination: The pigments fluoresce after they are extracted in an organic solvent and then excited by ligth of specific wavelengths. The fluoresced light emitted by samole then is filtered selectively to obtain the peak, which is detected by a sensitive photomultiplier tube. -HPLC determination: High Performance Liquid Cromatography.

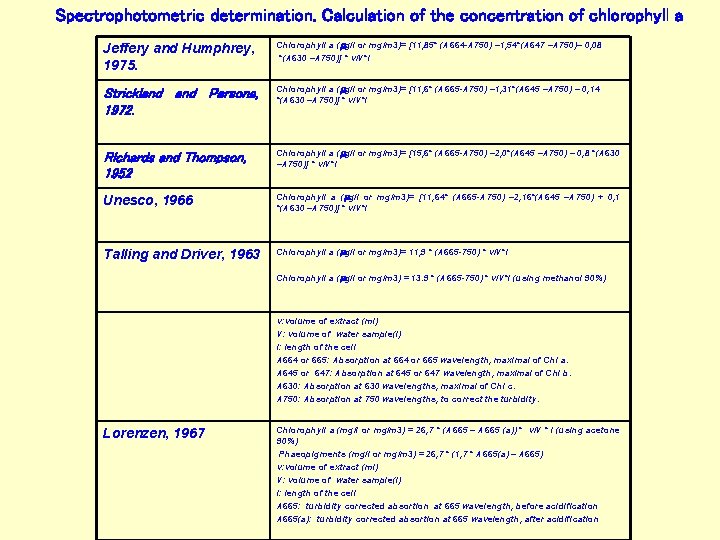
Spectrophotometric determination. Calculation of the concentration of chlorophyll a Jeffery and Humphrey, 1975. Chlorophyll a (mg/l or mg/m 3)= [11, 85* (A 664 -A 750) – 1, 54*(A 647 –A 750)– 0, 08 *(A 630 –A 750)] * v/V*l Strickland Parsons, 1972. Chlorophyll a (mg/l or mg/m 3)= [11, 6* (A 665 -A 750) – 1, 31*(A 645 –A 750) – 0, 14 *(A 630 –A 750)] * v/V*l Richards and Thompson, 1952 Chlorophyll a (mg/l or mg/m 3)= [15, 6* (A 665 -A 750) – 2, 0*(A 645 –A 750) – 0, 8 *(A 630 –A 750)] * v/V*l Unesco, 1966 Chlorophyll a (mg/l or mg/m 3)= [11, 64* (A 665 -A 750) – 2, 16*(A 645 –A 750) + 0, 1 *(A 630 –A 750)] * v/V*l Talling and Driver, 1963 Chlorophyll a (mg/l or mg/m 3)= 11, 9 * (A 665 -750) * v/V*l Chlorophyll a (mg/l or mg/m 3) = 13. 9 * (A 665 -750) * v/V*l (using methanol 90%) v: volume of extract (ml) V: volume of water sample(l) l: length of the cell A 664 or 665: Absorption at 664 or 665 wavelength, maximal of Chl a. A 645 or 647: Absorption at 645 or 647 wavelength, maximal of Chl b. A 630: Absorption at 630 wavelengths, maximal of Chl c. A 750: Absorption at 750 wavelengths, to correct the turbidity. Lorenzen, 1967 Chlorophyll a (mg/l or mg/m 3) = 26, 7 * (A 665 – A 665 (a)) * v/V * l (using acetone 90%) Phaeopigments (mg/l or mg/m 3) = 26, 7 * (1, 7 * A 665(a) – A 665) v: volume of extract (ml) V: volume of water sample(l) l: length of the cell A 665: turbidity corrected absortion at 665 wavelength, before acidification A 665(a): turbidity corrected absortion at 665 wavelength, after acidification