Translational and Personalized Medicine Initiative Quality of Care



![TPMI Research Day [Oct. 8, 2015] TPMI Research Day [Oct. 8, 2015]](https://slidetodoc.com/presentation_image_h2/f87ab2c84a907f531a158c949a081c90/image-11.jpg)

- Slides: 18

Translational and Personalized Medicine Initiative: Quality of Care Project Report

Drug Utilization Working Group – Overarching goals • To facilitate and advise on Quality of Care projects investigating: • drug utilization • drug safety • cost-effectiveness TPMI Research Day [Oct. 8, 2015]

Drug Utilization Working Group – Overarching goals • Projects will focus on medications known to be associated with: • • suboptimal utilization adverse reactions poor treatment response high levels of healthcare utilization & costs TPMI Research Day [Oct. 8, 2015]

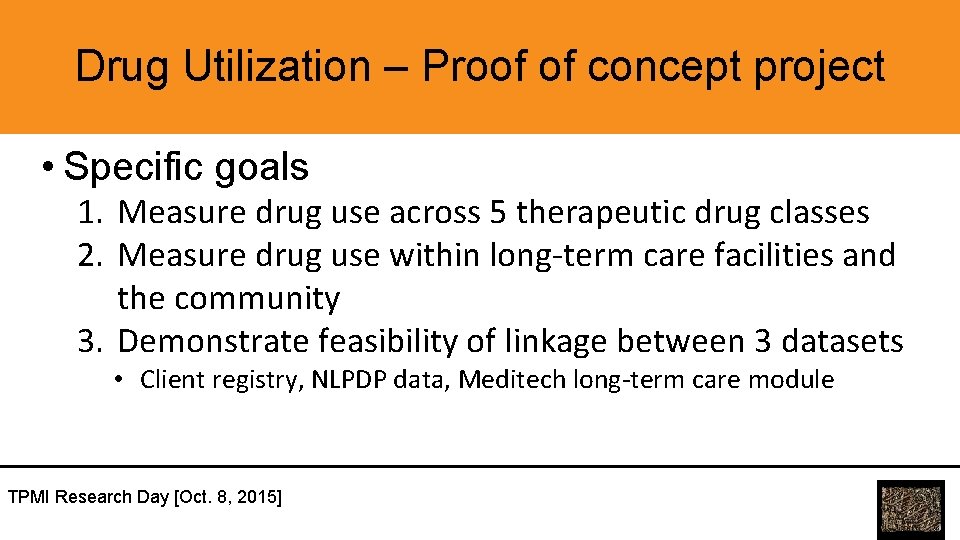
Drug Utilization – Proof of concept project • Specific goals 1. Measure drug use across 5 therapeutic drug classes 2. Measure drug use within long-term care facilities and the community 3. Demonstrate feasibility of linkage between 3 datasets • Client registry, NLPDP data, Meditech long-term care module TPMI Research Day [Oct. 8, 2015]

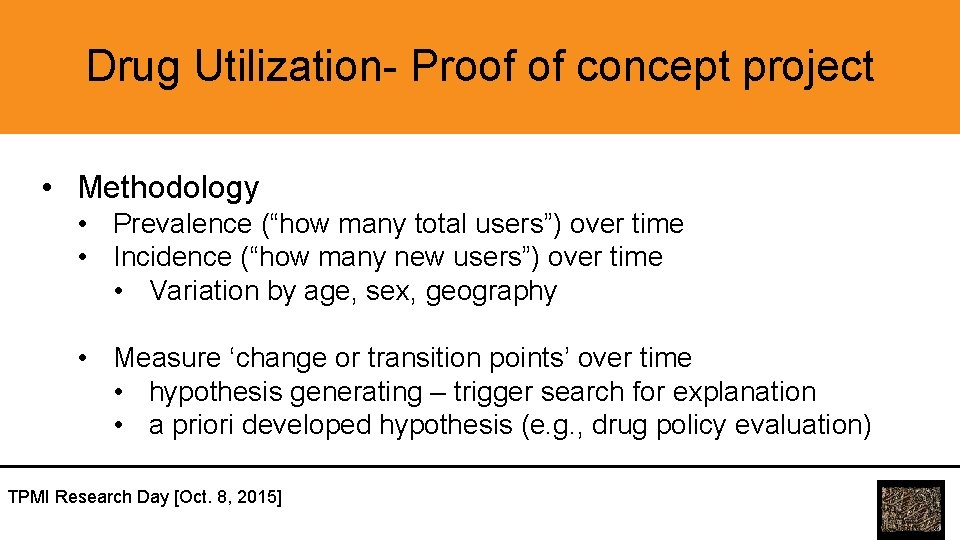
Drug Utilization- Proof of concept project • Methodology • Prevalence (“how many total users”) over time • Incidence (“how many new users”) over time • Variation by age, sex, geography • Measure ‘change or transition points’ over time • hypothesis generating – trigger search for explanation • a priori developed hypothesis (e. g. , drug policy evaluation) TPMI Research Day [Oct. 8, 2015]

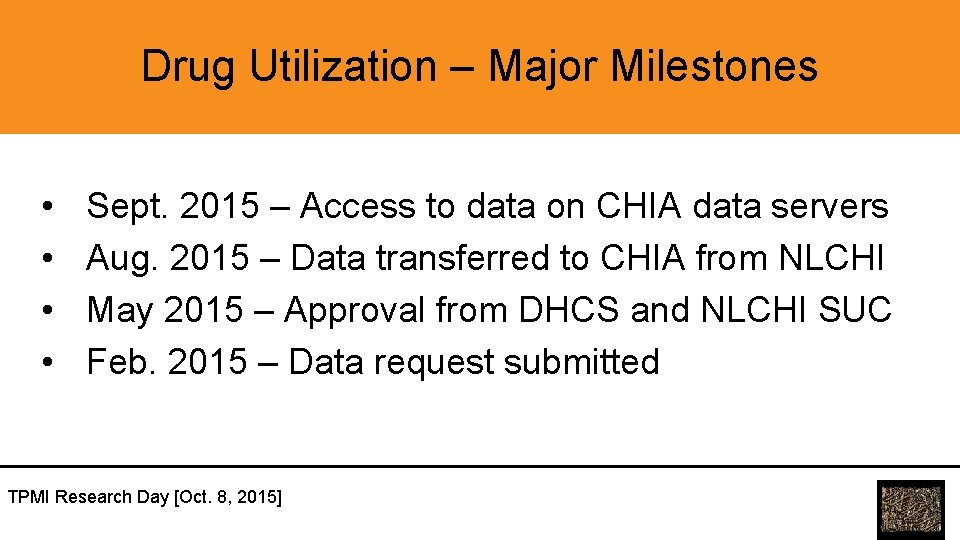
Drug Utilization – Major Milestones • • Sept. 2015 – Access to data on CHIA data servers Aug. 2015 – Data transferred to CHIA from NLCHI May 2015 – Approval from DHCS and NLCHI SUC Feb. 2015 – Data request submitted TPMI Research Day [Oct. 8, 2015]

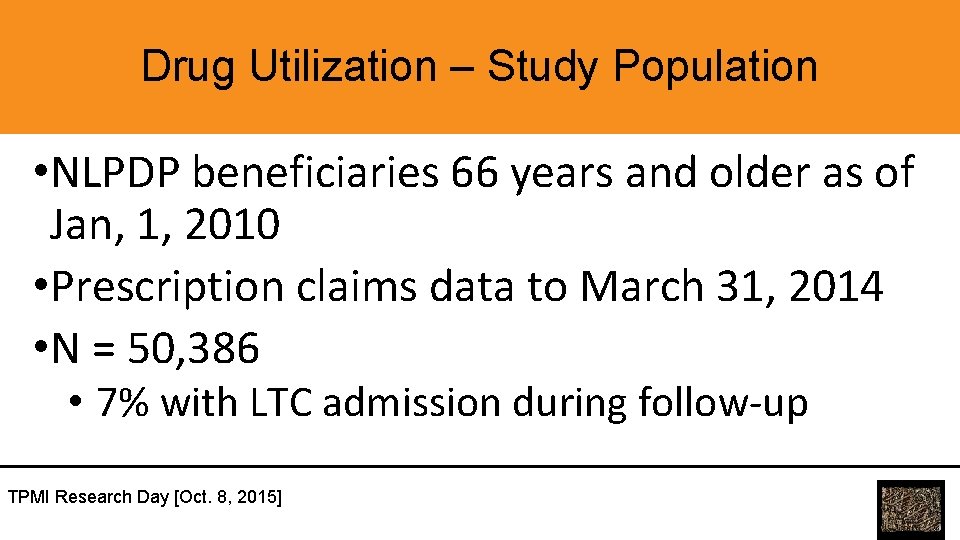
Drug Utilization – Study Population • NLPDP beneficiaries 66 years and older as of Jan, 1, 2010 • Prescription claims data to March 31, 2014 • N = 50, 386 • 7% with LTC admission during follow-up TPMI Research Day [Oct. 8, 2015]

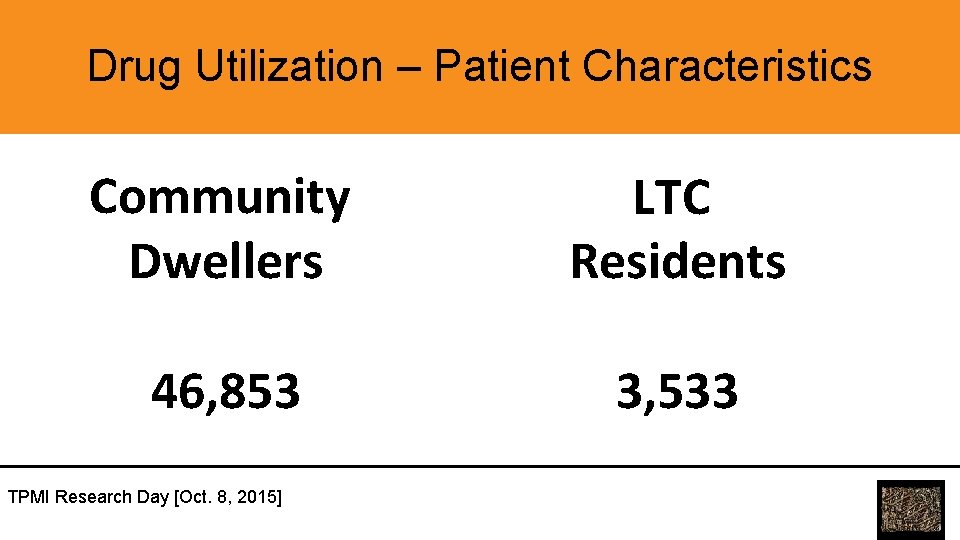
Drug Utilization – Patient Characteristics Community Dwellers LTC Residents 46, 853 3, 533 TPMI Research Day [Oct. 8, 2015]

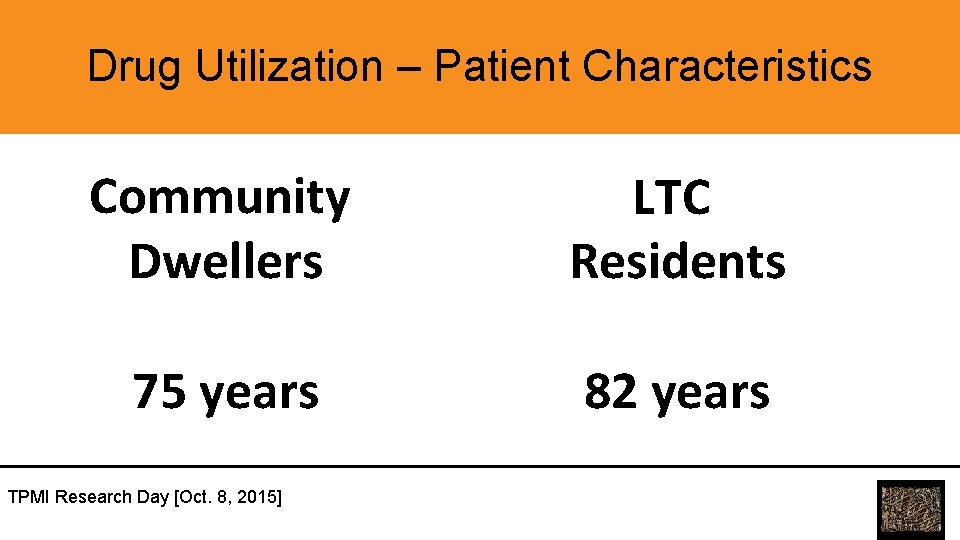
Drug Utilization – Patient Characteristics Community Dwellers LTC Residents 75 years 82 years TPMI Research Day [Oct. 8, 2015]

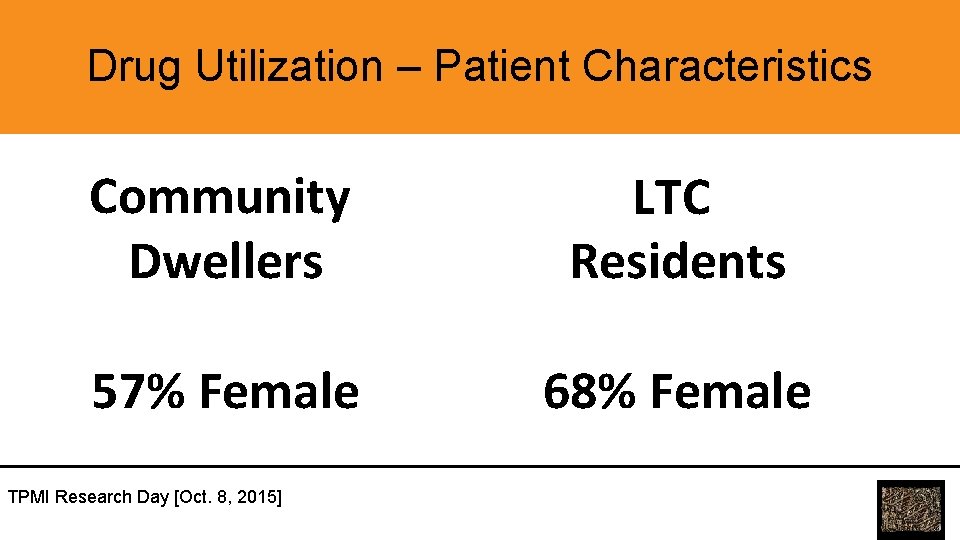
Drug Utilization – Patient Characteristics Community Dwellers LTC Residents 57% Female 68% Female TPMI Research Day [Oct. 8, 2015]
![TPMI Research Day Oct 8 2015 TPMI Research Day [Oct. 8, 2015]](https://slidetodoc.com/presentation_image_h2/f87ab2c84a907f531a158c949a081c90/image-11.jpg)
TPMI Research Day [Oct. 8, 2015]

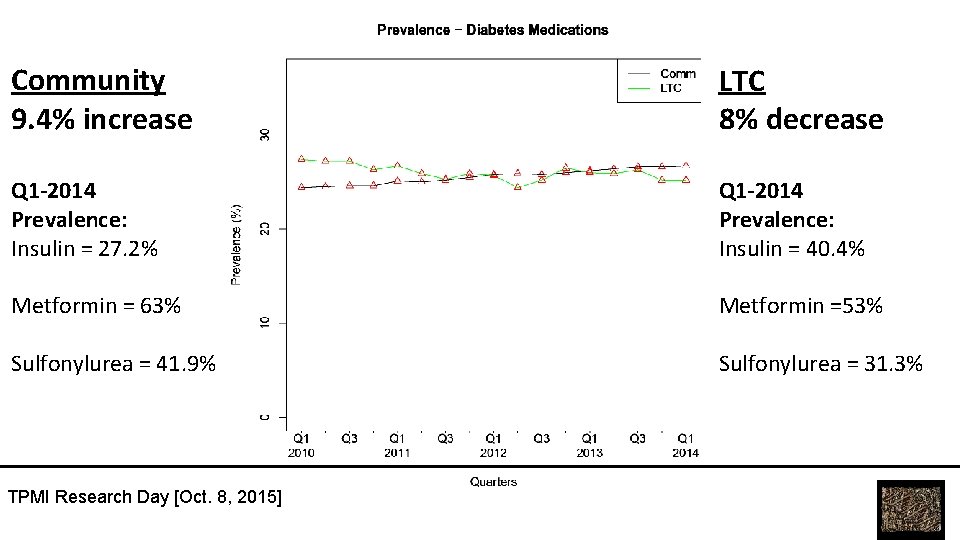
Community 9. 4% increase LTC 8% decrease Q 1 -2014 Prevalence: Insulin = 27. 2% Q 1 -2014 Prevalence: Insulin = 40. 4% Metformin = 63% Metformin =53% Sulfonylurea = 41. 9% Sulfonylurea = 31. 3% TPMI Research Day [Oct. 8, 2015]

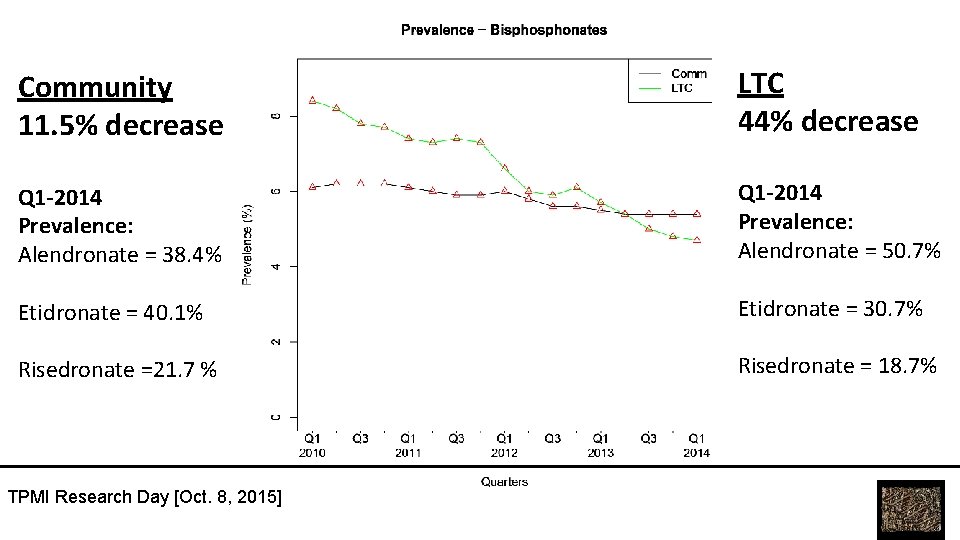
Community 11. 5% decrease LTC 44% decrease Q 1 -2014 Prevalence: Alendronate = 38. 4% Q 1 -2014 Prevalence: Alendronate = 50. 7% Etidronate = 40. 1% Etidronate = 30. 7% Risedronate =21. 7 % Risedronate = 18. 7% TPMI Research Day [Oct. 8, 2015]

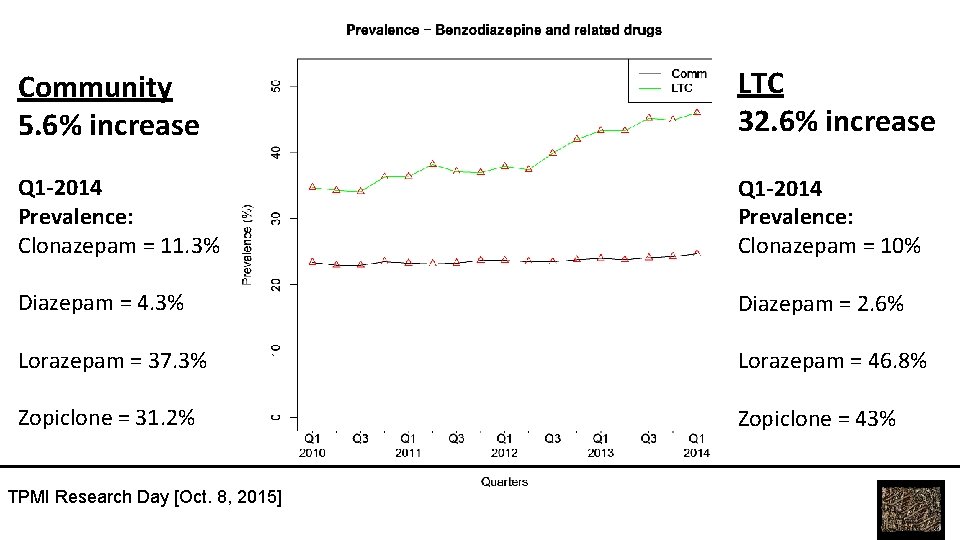
Community 5. 6% increase LTC 32. 6% increase Q 1 -2014 Prevalence: Clonazepam = 11. 3% Q 1 -2014 Prevalence: Clonazepam = 10% Diazepam = 4. 3% Diazepam = 2. 6% Lorazepam = 37. 3% Lorazepam = 46. 8% Zopiclone = 31. 2% Zopiclone = 43% TPMI Research Day [Oct. 8, 2015]

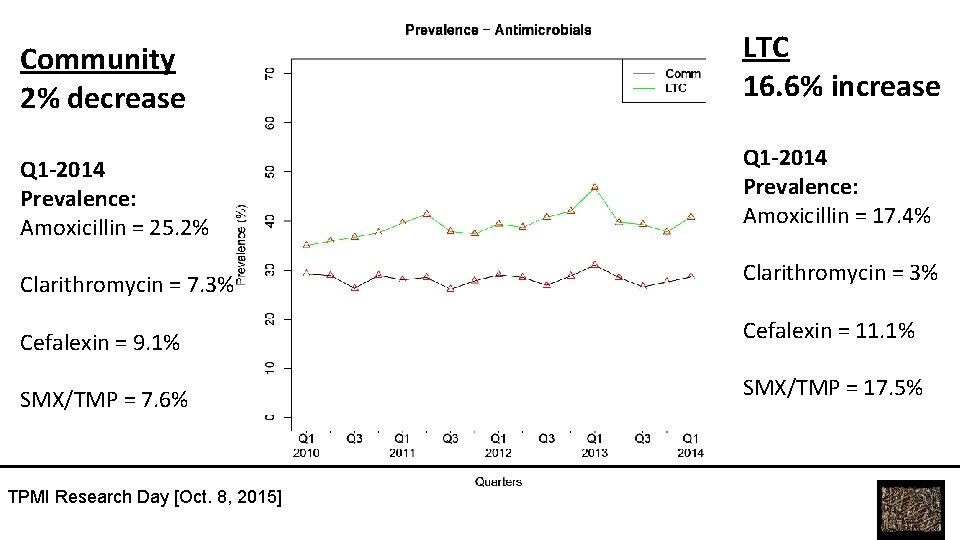
Community 2% decrease LTC 16. 6% increase Q 1 -2014 Prevalence: Amoxicillin = 25. 2% Q 1 -2014 Prevalence: Amoxicillin = 17. 4% Clarithromycin = 7. 3% Clarithromycin = 3% Cefalexin = 9. 1% Cefalexin = 11. 1% SMX/TMP = 7. 6% SMX/TMP = 17. 5% TPMI Research Day [Oct. 8, 2015]

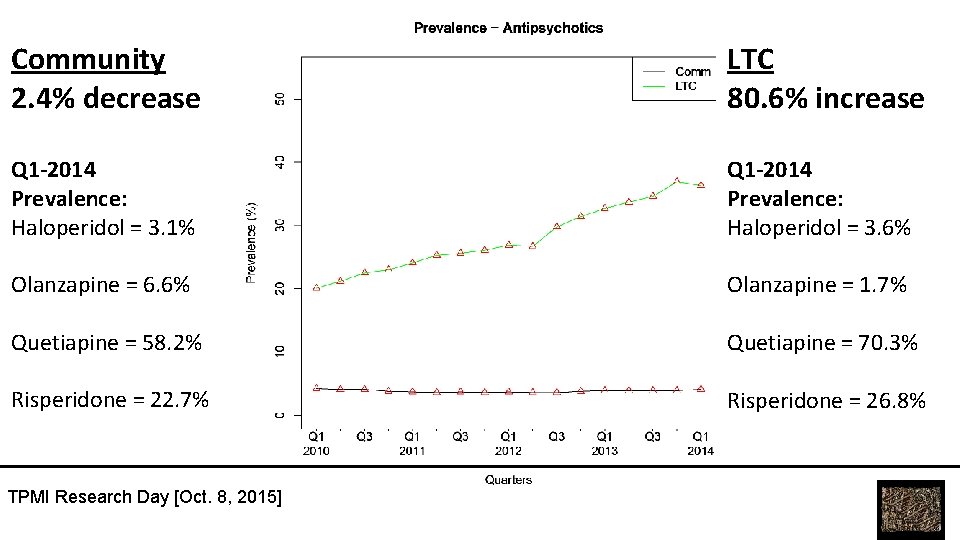
Community 2. 4% decrease LTC 80. 6% increase Q 1 -2014 Prevalence: Haloperidol = 3. 1% Q 1 -2014 Prevalence: Haloperidol = 3. 6% Olanzapine = 6. 6% Olanzapine = 1. 7% Quetiapine = 58. 2% Quetiapine = 70. 3% Risperidone = 22. 7% Risperidone = 26. 8% TPMI Research Day [Oct. 8, 2015]

Drug Utilization – Next Phases of Current Project 1. 2. 3. 4. Measure rates for individual medications Measure ‘change points’ over time Measure variation over age, sex, geography Evaluate association between medication use and health outcomes and health resource utilization • Fragility fractures • Hospital admissions • Mortality TPMI Research Day [Oct. 8, 2015]

Drug Utilization – Potential Future Projects 1. Plan and evaluate interventions to optimize drug utilization • Policy recommendations • Academic detailing service 2. Evaluate the cost-effectiveness of alternative approaches to optimizing drug utilization 3. Integrate drug use data from the Pharmacy Network TPMI Research Day [Oct. 8, 2015]
 Medicine
Medicine Duke translational medicine institute
Duke translational medicine institute Primary secondary and tertiary health care
Primary secondary and tertiary health care Mqii
Mqii Workforce data quality initiative
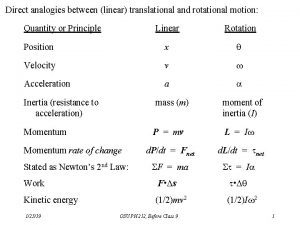
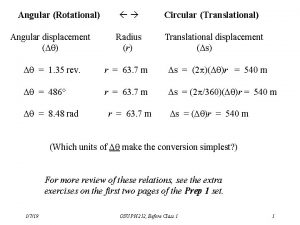
Workforce data quality initiative Torque and moment of inertia
Torque and moment of inertia Post translational and co translation
Post translational and co translation Rotational and linear motion analogies
Rotational and linear motion analogies Ap physics unit 7 mcq part a
Ap physics unit 7 mcq part a Sigir 2018
Sigir 2018 Personalised mobile search engine
Personalised mobile search engine Pentaho personalized demo
Pentaho personalized demo Personalized patient education
Personalized patient education Contextual bandits for personalized recommendation
Contextual bandits for personalized recommendation Personalized recommendations
Personalized recommendations Personalized navigation
Personalized navigation Personal statement first draft
Personal statement first draft All to one reduction
All to one reduction Trait approach leadership
Trait approach leadership