Translational and Clinical Research Project LSUHSC Research Council























- Slides: 25

Translational and Clinical Research Project LSUHSC Research Council

Translational Claude Burgoyne, M. D. - Chairman Paul Fidel, Ph. D James Hardy Jean Jacob, Ph. D. Michal Jazwinski, Ph. D. Alistar Ramsay, Ph. D. LSUHSC Research Council

Translation Research Opportunities; Approach • Identity NIH funded investigators -3 questions by email • Identify Center/Program Directors • Identify Departmental Chairmen -3 questions by e-mail • Compile responses in summary files

Translation Research Opportunities; Recommentdations • Create office of Translational research • Seek a dynamic Director • Focus on two areas of endeavor -TR initiatives within each Center and Program -Basic science forward initiative • TR Initiatives within Center and Program -Why start here?

Translation Research opportunities; Basic Science Forward Initiative • Make TR office the center of scientific interaction • Consider including Inter-disciplinary Research • Foster three forms of interaction 1. Basic scientist seeking clinician 2. Clinician seeking basic scientist 3. Basic seeking basic scientist • Three goals of Initiative 1. Publicize unique resources of LSUHSC 2. Create accessible database of scientists 3. Foster inter-action through personal inter-action

Interdisciplinary Greg Bagby, Ph. D. James Cairo, Ph. D. William Chilian, Ph. D. - Chairman Paul Fidel, Ph. D. Stephen Lanier, Ph. D. Carol Mason, M. D. LSUHSC Research Council

Interdisciplinary Research: Recommendations • New leadership needed in clinical departments to change the culture to active support for research • Sharing of IDC Recovery between schools and departments in multi school and muiti-department grants • Creation of an Office of Interdisciplinary Research • Increased allocation of IDC Recovery to Departments with Interdisciplinary research support • Change the culture of the HSC administration from a "can't do" to a "can do" attitude

Clinical Research in the HCSD Michael Butler, M. D. William Cassidy, M. D. Bennett De. Boisblanc, M. D. Kathy Hebert, M. D. Ronald Horswell, Ph. D. John Hunt, M. D. - Chairman Demetruis Porche, Ph. D. Patricia Snyder, Ph. D. Mark Warner, Ph. D. LSUHSC Research Council

CLINICAL RESEARCH AND THE HCSD: Recommendations IRB • Increase IRB Support Staff; Increase frequency of meetings to 2 X/month • Internet-based processing of SAE's amendments, etc • Have IRB members from all institutions subject to IRB cognizance • Consider IRB reciprocity with other institutions, e. g Tulane, Ochsner • Explore use of "Central IRB" for multi-institutional projects

CLINICAL RESEARCH AND THE HCSD: Recommendations FINANCIAL • Supplement investigator's salaries for doing funded research • Conduct funds-flow analysis (Hospitals to LSU and LSU to Hospitals) • Research money generated at an institution should stay there • Permit competitive salaries for Research Coordinators • Improve COLA's for employees • Develop on-line access by investigators to financial data • Develop "internal CRO"/Facilitator Office at each hospital

CLINICAL RESEARCH AND THE HCSD: Recommendations PEOPLE AND INFRASTRUCTURE • Recruit personnel (physicians, nurses, etc) with research experience • Provide education and training to interested personnel in satellite hospitals • Utilize a single electronic medical record system throughout the HCSD

Clinical Trials Office Luis Balart, M. D. Jill Gilbert, M. D. David Martin, M. D. Steve Nelson, M. D. - Chairman Mark Townsend, M. D. Michele Zembo, M. D. LSUHSC Research Council

Clinical Trials Office • The mission of the Clinical Trials Office is to organize and enhance operational processes that support clinical research and facilitate the timely initiation, execution, management, and completion of clinician trials at the LSU Health Sciences Center. • The Clinical Trials Office will function as a single point-of-contact for all aspects of clinical trials research. • The primary goals of the Clinical Trials Office are to offer a full spectrum of services that include 1) planning and conducting high quality clinical research trials, 2) enhancing administrative efficiency, and 3) assisting in the development and implementation of policies and procedures that assure compliance with prevailing regulations that govern the conduct of research in humans.

These services are focused in five major areas: 1. Administrative 2. Regulatory Compliance 3. Specialized assistance with investigator initiated studies 4. Education/Training 5. Quality Control/Quality Assurance

The Clinical Trials Office will help LSUHSC meet its mission goals of excellence in patient care, education, research, and community service. LSUHSC is committed to providing world class patient care with innovative therapies by facilitating access to cutting edge clinical research opportunities.

Human Bio-specimen Core Kathleen Dunlap, M. D. Kenneth Fallon, M. D. Jong Kim, Ph. D. Sam Parthasarathy, Ph. D. - Chairman Madhwa Raj, Ph. D. Eugene Woltering, M. D. LSUHSC Research Council

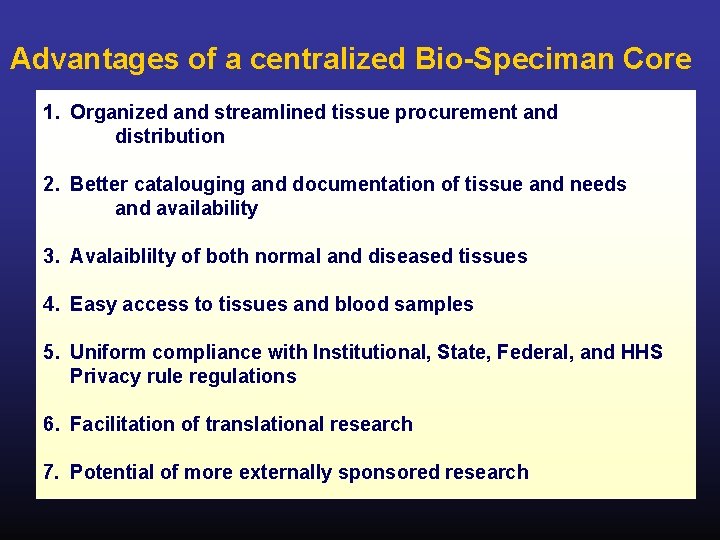
Advantages of a centralized Bio-Speciman Core 1. Organized and streamlined tissue procurement and distribution 2. Better catalouging and documentation of tissue and needs and availability 3. Avalaiblilty of both normal and diseased tissues 4. Easy access to tissues and blood samples 5. Uniform compliance with Institutional, State, Federal, and HHS Privacy rule regulations 6. Facilitation of translational research 7. Potential of more externally sponsored research

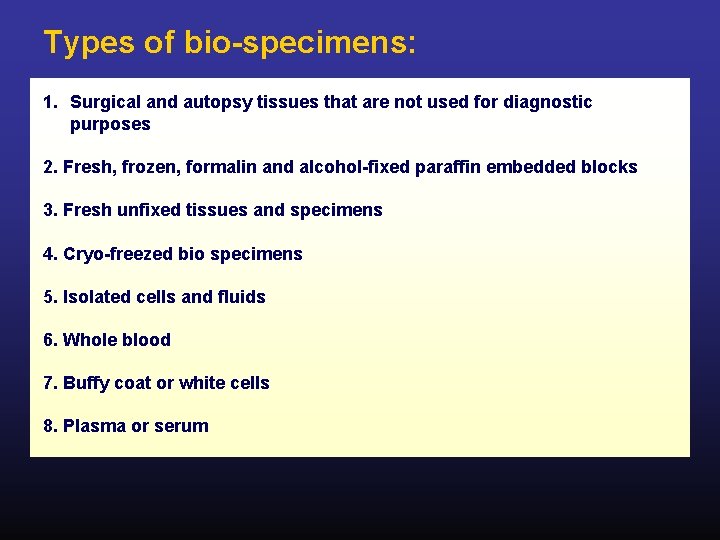
Types of bio-specimens: 1. Surgical and autopsy tissues that are not used for diagnostic purposes 2. Fresh, frozen, formalin and alcohol-fixed paraffin embedded blocks 3. Fresh unfixed tissues and specimens 4. Cryo-freezed bio specimens 5. Isolated cells and fluids 6. Whole blood 7. Buffy coat or white cells 8. Plasma or serum

Recommendations: • Of the existing centers at LSUHSC, the Cancer Center Core appears to be well developed and already in place. Despite a few minor deficiencies, the Cancer Center core is better suited to serve the needs of the campus. It is recommended that this core is further developed to utilize the procurement of normal tissues and blood samples. The Cancer Center Core would thus become the centralized and main bio-specimen core of the campus. • It is recommended that a “wet laboratory” is setup to process blood samples to isolate cells, plasma/serum and to dissect out tissues. • It is recommended that the Cancer Center Core incorporate additional members in their committee who would advise on the procurement of control and normal samples. • It is recommended that specific people/protocols are identified for investigators to contact the core regarding the need of samples. • Future directions: • The Bio-specimen core could be integrated with a “clinical trials” office. • Novel cells derived from tissues could be immortalized (Technology Transfer)

Education, Training and Mentoring John Burgess, D. S. S. Kurt Varner, Ph. D. James Diaz, M. D. Connie Romaine, RN, MN Judd Shellito, M. D. Warren Summer, M. D. - Chairman LSUHSC Research Council

Education, Training and Mentoring for Clinical Research: Recommendations • Promote clinical research • Identify potential clinician researchers • Attract potential clinical researchers • Design an Optimal Core Curriculum (Certificate with 9 -12 graduate credit hours) • Supplement Certificate and Web-based programs to MS degree in Clinical Research

Education, Training and Mentoring for Clinical Research: Recommendations • Provide ongoing support (admin. , space, funds) to new Clinician-researchers and their mentors • Provide ongoing support for mentoring of clinician-researchers • Jump-start and obtain external funds for program • Manage resistance to change

Incentives and Disincentives John Estrada, M. D. Steve Nelson, M. D. Augusto Ochoa, M. D. - Chairman LSUHSC Research Council

Incentives/Disincentives in Clinical Research: Recommendations Immediate: • Create a plan of monetary and promotion incentives for clinician-scientists • Establish site for development of clinical/translational research • Establish research experience as major criterion for hiring faculty/chairs Three to five years: • Strengthen existing clinician-scientists enabling them to mentor residents, fellows and junior faculty • Develop a Clinical Trials Office

Louisiana Regional Translational Research Center (LARTRC) • NIH application for planning grant submitted today ($150, 000 x 1 yr) • Consortium of: LSUHSC-NO, LSUHSC-S, Pennington Research Center, HCSD • Other local institutions and major pharmaceutical companies to be considered • RFA for five year, $7 million grant due in 2006 • Vision for seamless integration of translational research support and infrastructure • Priority areas to be determined, eg obesity/diabetes, cardiovascular disease, etc • Governance by Executive Committee appointed by LSU President
 Lsuhsc redcap
Lsuhsc redcap New innovations lsuhsc
New innovations lsuhsc Peoplesoft lsuhsc shreveport
Peoplesoft lsuhsc shreveport Lsuhsc pediatrics
Lsuhsc pediatrics Lsuhsc school of public health
Lsuhsc school of public health Aquifer lsuhsc
Aquifer lsuhsc Primary control vs secondary control
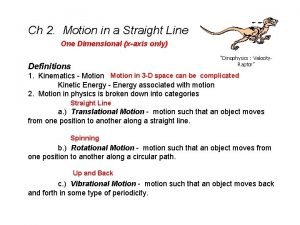
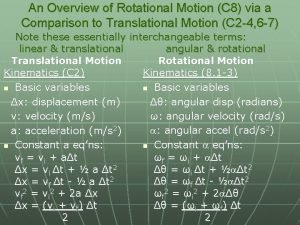
Primary control vs secondary control Translational vs rotational motion
Translational vs rotational motion Post translational and co translation
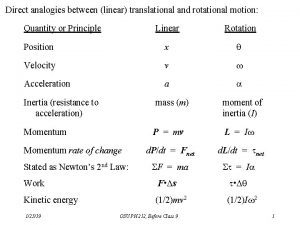
Post translational and co translation Analogy between linear and rotational motion
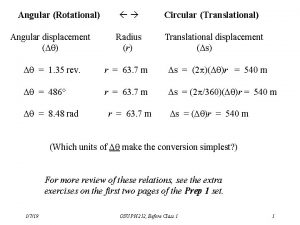
Analogy between linear and rotational motion Ap physics 1 unit 7 mcq
Ap physics 1 unit 7 mcq Translational equilibrium?
Translational equilibrium? Lj wei harvard
Lj wei harvard Translational kinetic energy
Translational kinetic energy Oscillatory motion
Oscillatory motion Translational kinetic energy
Translational kinetic energy Translational kinetic energy formula
Translational kinetic energy formula Translational motion definition
Translational motion definition Translational kinetic energy formula
Translational kinetic energy formula Translational vs rotational motion
Translational vs rotational motion Translational criminology
Translational criminology Rolling with slipping
Rolling with slipping Translational speed formula
Translational speed formula Answer
Answer Duke translational medicine institute
Duke translational medicine institute Translational slide
Translational slide