The Third Law of Thermodynamics If the entropy

- Slides: 8

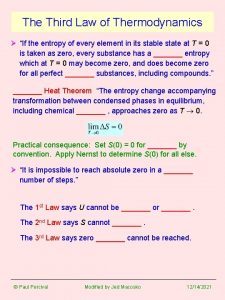
The Third Law of Thermodynamics Ø “If the entropy of every element in its stable state at T = 0 is taken as zero, every substance has a _______ entropy which at T = 0 may become zero, and does become zero for all perfect _______ substances, including compounds. ” _______ Heat Theorem “The entropy change accompanying transformation between condensed phases in equilibrium, including chemical _______ , approaches zero as T 0. Practical consequence: Set S(0) = 0 for _______ by convention. Apply Nernst to determine S(0) for all else. Ø “It is impossible to reach absolute zero in a _______ number of steps. ” The 1 st Law says U cannot be _______ or _______. The 2 nd Law says S cannot _______. The 3 rd Law says zero _______ cannot be reached. © Paul Percival Modified by Jed Macosko 11/6/2020

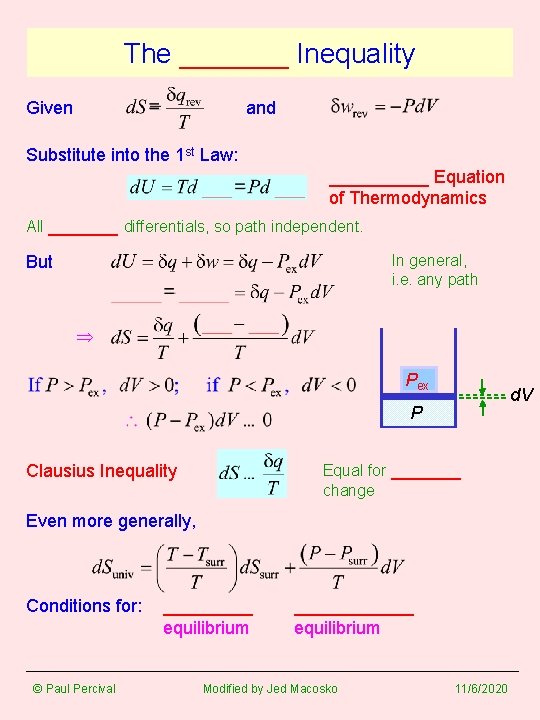
The _______ Inequality Given and Substitute into the 1 st Law: _____ Equation of Thermodynamics All _______ differentials, so path independent. In general, i. e. any path But Pex d. V P Equal for _______ change Clausius Inequality Even more generally, Conditions for: © Paul Percival _____ equilibrium ______ equilibrium Modified by Jed Macosko 11/6/2020

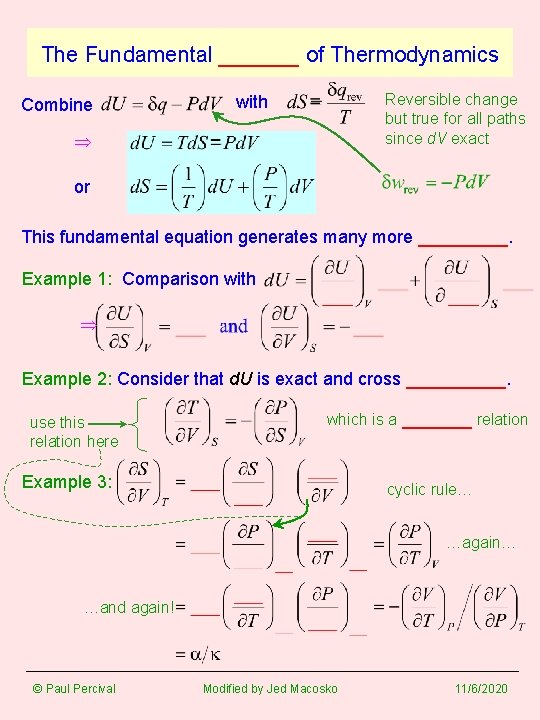
The Fundamental ______ of Thermodynamics Combine Reversible change but true for all paths since d. V exact with or This fundamental equation generates many more _____. Example 1: Comparison with Example 2: Consider that d. U is exact and cross _____. use this relation here which is a _______ relation Example 3: cyclic rule… …again… …and again! © Paul Percival Modified by Jed Macosko 11/6/2020

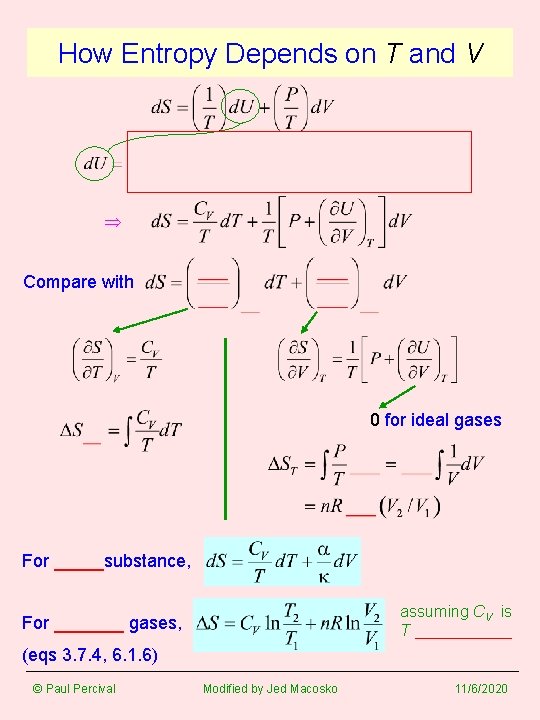
How Entropy Depends on T and V Compare with 0 for ideal gases For _____substance, assuming CV is T ______ For _______ gases, (eqs 3. 7. 4, 6. 1. 6) © Paul Percival Modified by Jed Macosko 11/6/2020

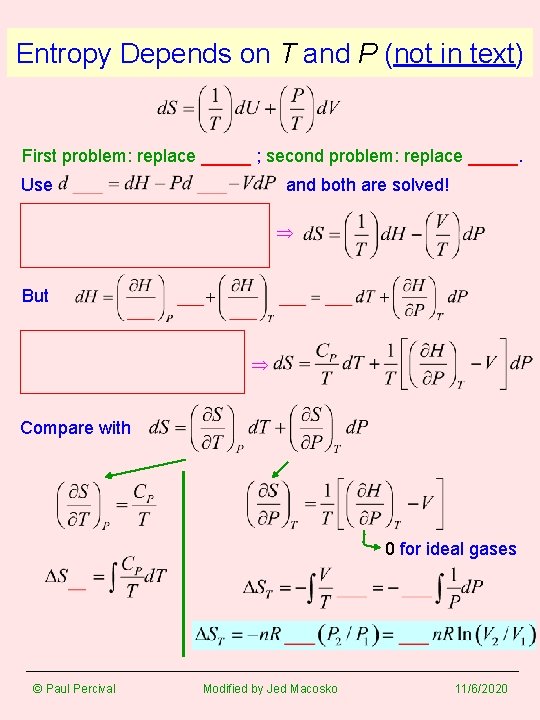
Entropy Depends on T and P (not in text) First problem: replace _____ ; second problem: replace _____. Use and both are solved! But Compare with 0 for ideal gases © Paul Percival Modified by Jed Macosko 11/6/2020

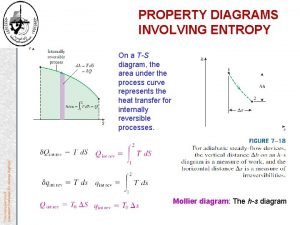
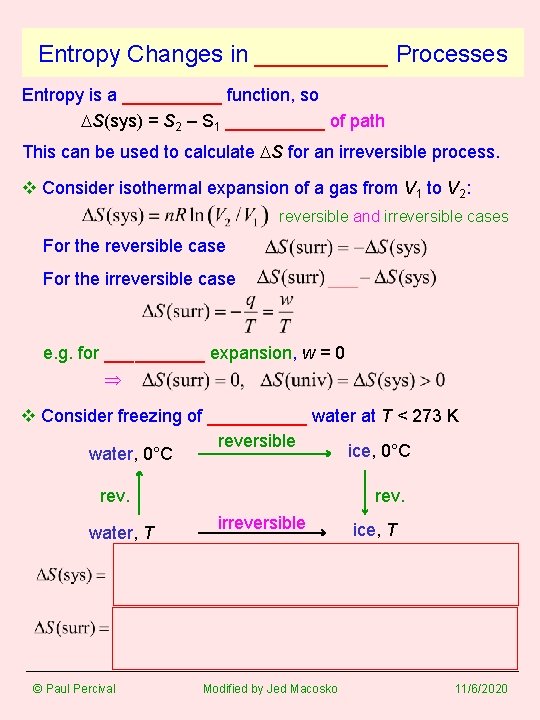
Entropy Changes in _____ Processes Entropy is a _____ function, so DS(sys) = S 2 – S 1 _____ of path This can be used to calculate DS for an irreversible process. v Consider isothermal expansion of a gas from V 1 to V 2: reversible and irreversible cases For the reversible case For the irreversible case e. g. for _____ expansion, w = 0 v Consider freezing of _____ water at T < 273 K reversible ice, 0°C water, 0°C rev. water, T © Paul Percival rev. irreversible Modified by Jed Macosko ice, T 11/6/2020

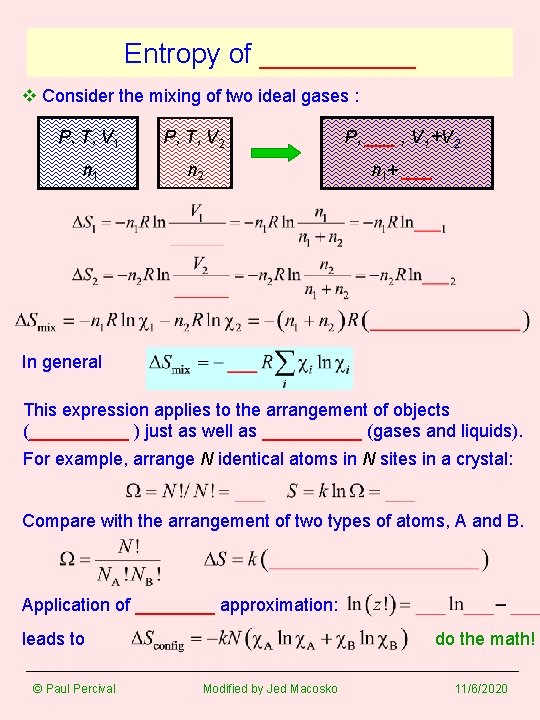
Entropy of _____ v Consider the mixing of two ideal gases : P, T, V 1 P, T, V 2 P, ___ , V 1+V 2 n 1 n 2 n 1+ ___ In general This expression applies to the arrangement of objects (_____ ) just as well as _____ (gases and liquids). For example, arrange N identical atoms in N sites in a crystal: Compare with the arrangement of two types of atoms, A and B. Application of ____ approximation: leads to © Paul Percival do the math! Modified by Jed Macosko 11/6/2020

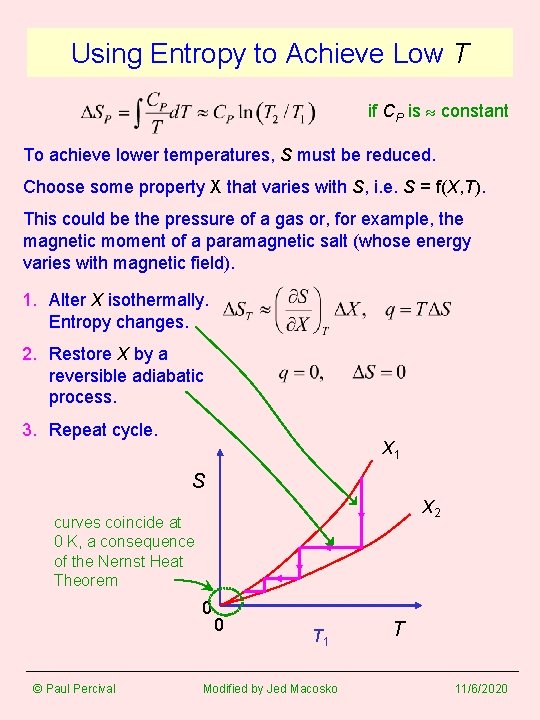
Using Entropy to Achieve Low T if CP is constant To achieve lower temperatures, S must be reduced. Choose some property X that varies with S, i. e. S = f(X, T). This could be the pressure of a gas or, for example, the magnetic moment of a paramagnetic salt (whose energy varies with magnetic field). 1. Alter X isothermally. Entropy changes. 2. Restore X by a reversible adiabatic process. 3. Repeat cycle. X 1 S X 2 curves coincide at 0 K, a consequence of the Nernst Heat Theorem 0 © Paul Percival 0 T 1 Modified by Jed Macosko T 11/6/2020