THE THEORY OF HYDROGEN BONDS HYDROGEN BONDS Allows

THE THEORY OF HYDROGEN BONDS
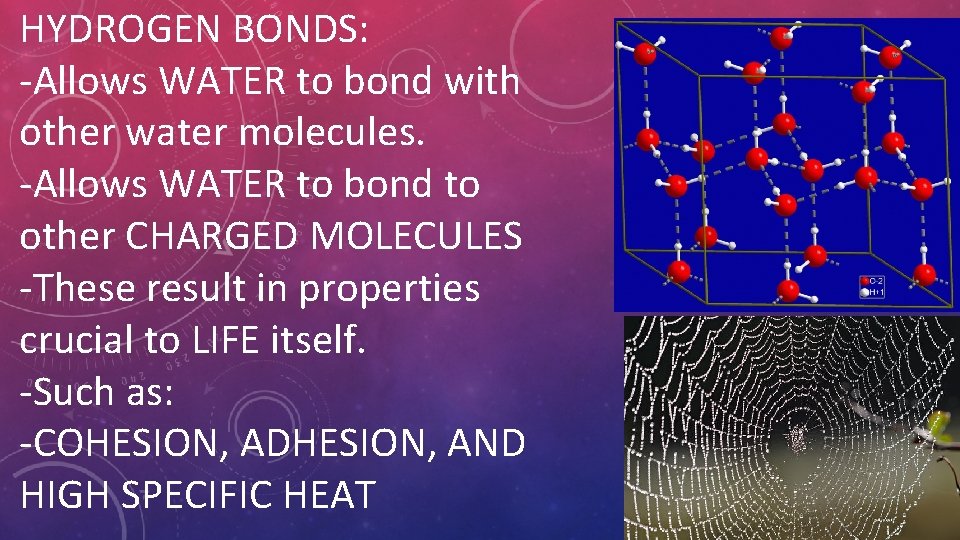
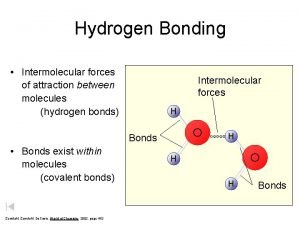
HYDROGEN BONDS: -Allows WATER to bond with other water molecules. -Allows WATER to bond to other CHARGED MOLECULES -These result in properties crucial to LIFE itself. -Such as: -COHESION, ADHESION, AND HIGH SPECIFIC HEAT

We can’t directly observe HYDROGEN BONDS Hydrogen Bond are A PROPOSED EXPLANATION FOR WHY WATER HAS THESE PROPERTIES. Therefore…HYDROGEN BONDING IS CONSIDERED A THEORY.

THE EXISTENCE OF HYDROGEN BONDS WILL (PROBABLY) NEVER BE PROVEN WITHOUT A DOUBT. THAT DOESN’T MEAN THEORY ISN’T CREDIBLE. Based on EVIDENCE this THEORY IS THE BEST EXPLANATION WE CURRENTLY HAVE FOR THE PROPERTIES OF WATER.
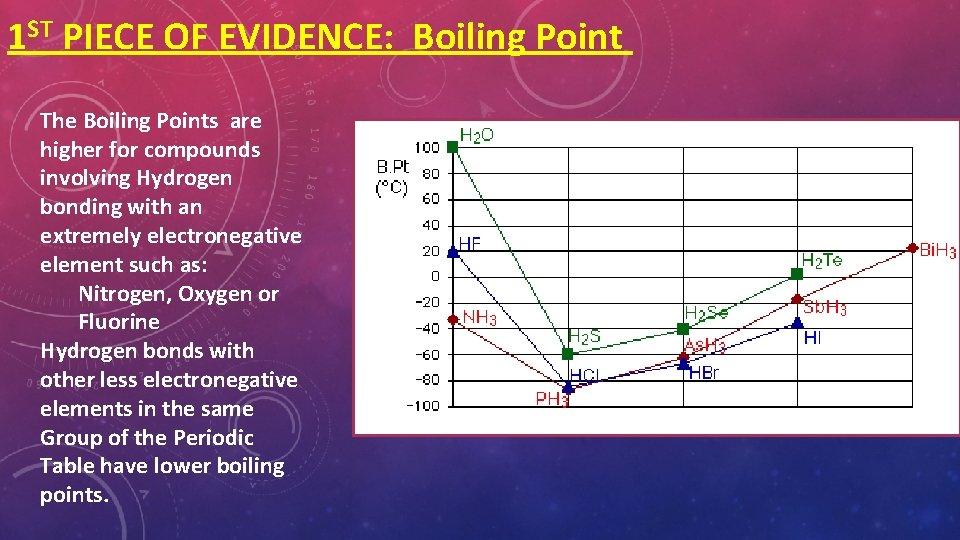
1 ST PIECE OF EVIDENCE: Boiling Point The Boiling Points are higher for compounds involving Hydrogen bonding with an extremely electronegative element such as: Nitrogen, Oxygen or Fluorine Hydrogen bonds with other less electronegative elements in the same Group of the Periodic Table have lower boiling points.

2 ND PIECE OF EVIDENCE: How easily DNA breaks apart: DNA denatures at extreme temperatures depending on how many Adenine-Thymine complementary base pairs are present compared to Guanine. Cytosine. This makes sense if you account for how many hydrogen bonds there are between complementary bases: G and C pair with 3 hydrogen bonds (and thus are a slightly stronger pair) A and T pair with 2 hydrogen bonds (and thus a slightly weaker pair).
3 rd PIECE OF EVIDENCE: Experimental Evidence: • NMR (nuclear magnetic resonance) • IR (infrared radiation) • X-Ray Diffraction • Neutron Diffractions

4 th PIECE OF EVIDENCE: Theoretical Simulation: • • Molecular Dynamics Monte Carlo Percolation Models Reversible Gelation Theory
- Slides: 8