The Earths Atmosphere What holds the Earths atmosphere




![Troposphere [tropopause at 8 -18 km, or 5 -11 miles] • Troposphere – contains Troposphere [tropopause at 8 -18 km, or 5 -11 miles] • Troposphere – contains](https://slidetodoc.com/presentation_image_h2/69bc9a1f68c1cc8a5850753612491eea/image-13.jpg)
![mesosphere Stratosphere [stratopause at 50 km, about 30 miles] – decrease in amount of mesosphere Stratosphere [stratopause at 50 km, about 30 miles] – decrease in amount of](https://slidetodoc.com/presentation_image_h2/69bc9a1f68c1cc8a5850753612491eea/image-14.jpg)



- Slides: 21

The Earth’s Atmosphere

What holds the Earth’s atmosphere to the planet? Y T I V A R G

Development of the Earth’s Atmosphere • Primordial atmosphere (4. 6 to 4. 0 bya) • Evolutionary atmosphere (4. 0 to 3. 3 bya) • The living atmosphere (3. 3 bya to 500 mya) • The modern atmosphere (500 mya to present)

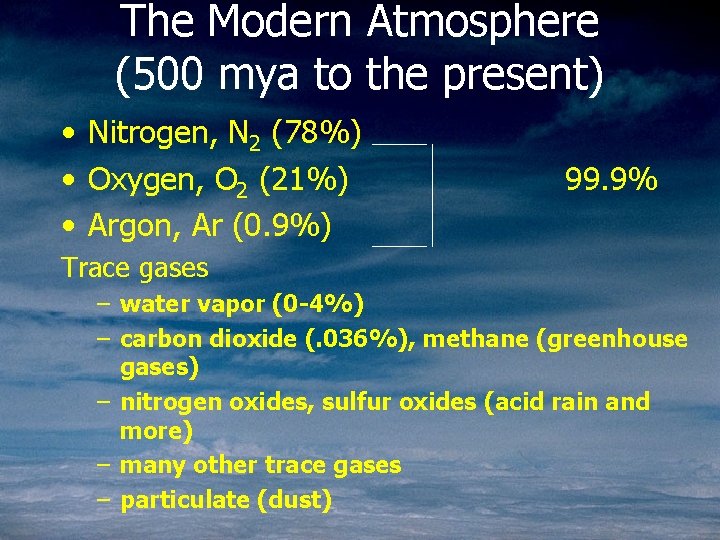
The Modern Atmosphere (500 mya to the present) • Nitrogen, N 2 (78%) • Oxygen, O 2 (21%) • Argon, Ar (0. 9%) 99. 9% Trace gases – water vapor (0 -4%) – carbon dioxide (. 036%), methane (greenhouse gases) – nitrogen oxides, sulfur oxides (acid rain and more) – many other trace gases – particulate (dust)

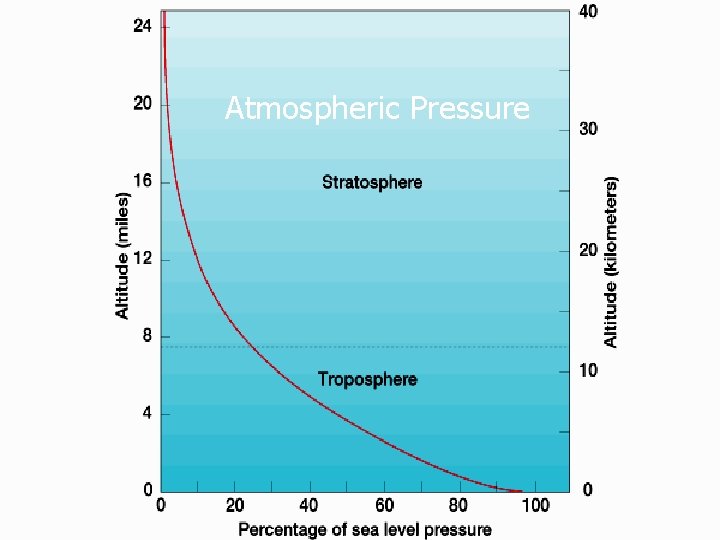
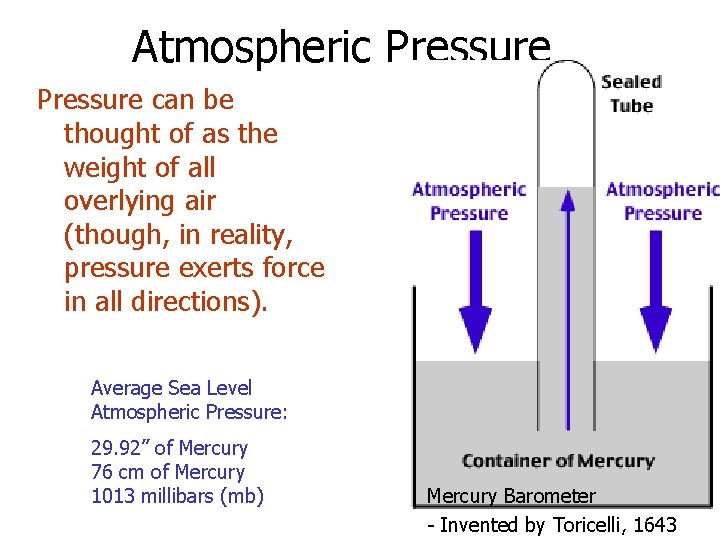
Atmospheric Pressure

Atmospheric Pressure can be thought of as the weight of all overlying air (though, in reality, pressure exerts force in all directions). Average Sea Level Atmospheric Pressure: 29. 92” of Mercury 76 cm of Mercury 1013 millibars (mb) Mercury Barometer - Invented by Toricelli, 1643

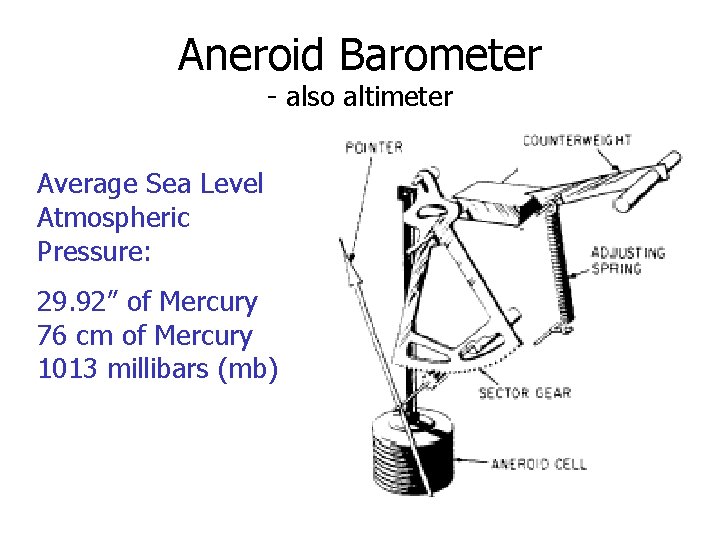
Aneroid Barometer - also altimeter Average Sea Level Atmospheric Pressure: 29. 92” of Mercury 76 cm of Mercury 1013 millibars (mb)

Atmospheric Pressure Is Related to Weather Conditions Less-Dense, Low Pressure Rises: Clouds and Stormy Weather More-Dense, High Pressure Air Sinks: Fair Weather

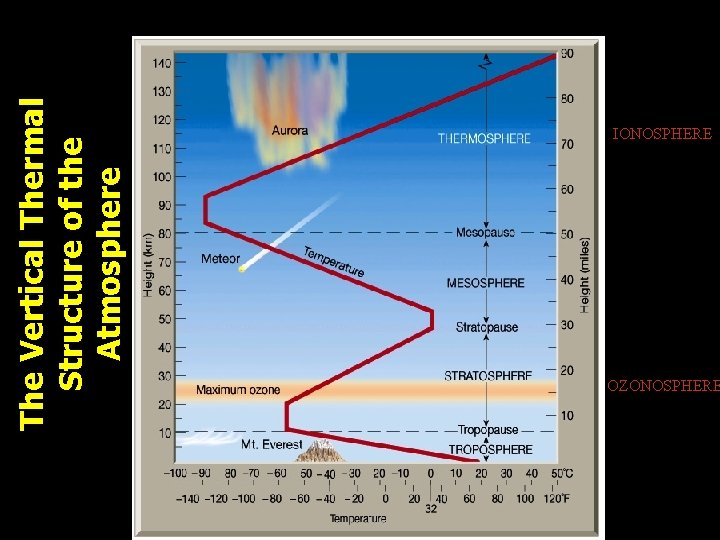
The Vertical Thermal Structure of the Atmosphere IONOSPHERE OZONOSPHERE

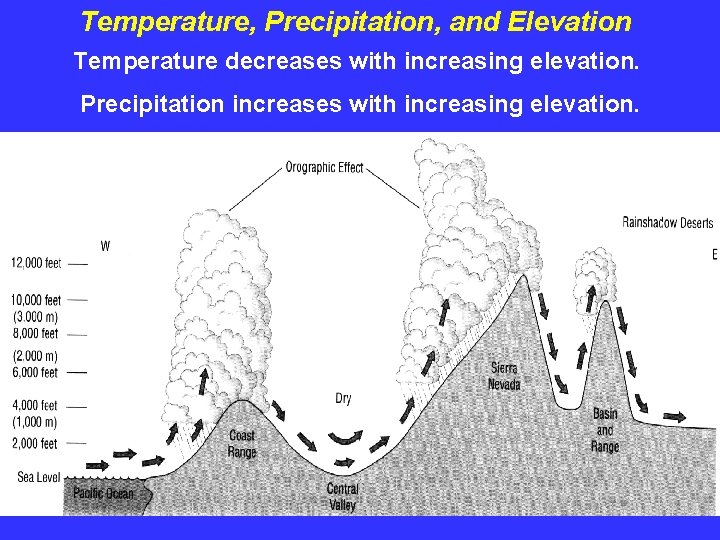
Temperature, Precipitation, and Elevation Temperature decreases with increasing elevation. Precipitation increases with increasing elevation.

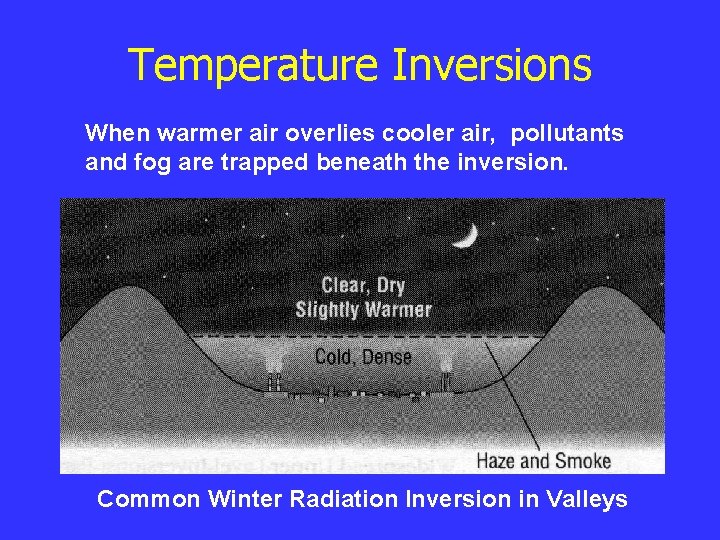
Temperature Inversions When warmer air overlies cooler air, pollutants and fog are trapped beneath the inversion. Common Winter Radiation Inversion in Valleys

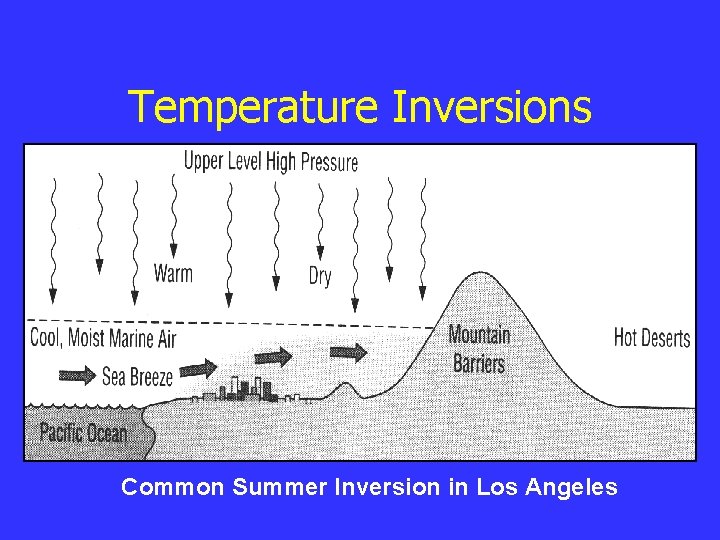
Temperature Inversions Common Summer Inversion in Los Angeles
![Troposphere tropopause at 8 18 km or 5 11 miles Troposphere contains Troposphere [tropopause at 8 -18 km, or 5 -11 miles] • Troposphere – contains](https://slidetodoc.com/presentation_image_h2/69bc9a1f68c1cc8a5850753612491eea/image-13.jpg)
Troposphere [tropopause at 8 -18 km, or 5 -11 miles] • Troposphere – contains 90% of the mass of the atmosphere – decrease of mass with altitude – mostly mixed gases (not layered) – clouds / weather layer – temperatures decrease with altitude - WHY?
![mesosphere Stratosphere stratopause at 50 km about 30 miles decrease in amount of mesosphere Stratosphere [stratopause at 50 km, about 30 miles] – decrease in amount of](https://slidetodoc.com/presentation_image_h2/69bc9a1f68c1cc8a5850753612491eea/image-14.jpg)
mesosphere Stratosphere [stratopause at 50 km, about 30 miles] – decrease in amount of gases with altitude – mixed gases (not stratified) except for ozone layer – temperatures increase with altitude [tropopause at 8 -18 km, or 5 -11 miles]

Aurora borealis / australis • The northern / southern lights • (click for video) (click for photos and legends) • Thermosphere and uppermost Mesosphere – solar wind (clouds of electrically charged particles) – Earth’s magnetic field directs them towards poles – excite oxygen (O) and nitrogen (N 2) ions in ionosphere emit light

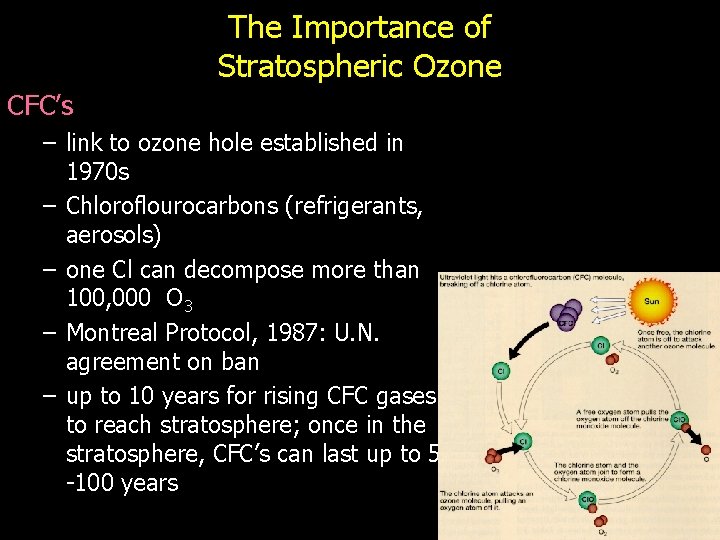
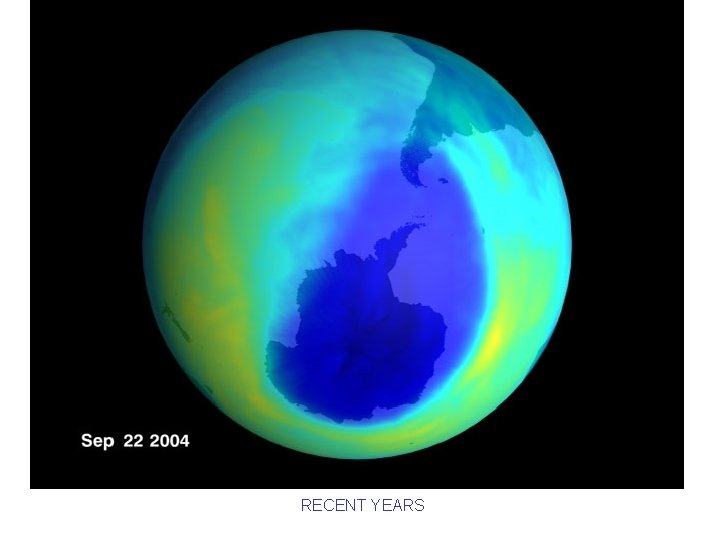
The Importance of Stratospheric Ozone • Ozone forms naturally in stratosphere light O 2 2 O then O + O 2 O 3 • UV radiation (sun) --> mutations – plankton reduced (food chain base), crops decline – weaker immune systems, skin cancer • Stratospheric ozone (O 3) absorbs UV rays O 3 O 2 + O

The Importance of Stratospheric Ozone CFC’s – link to ozone hole established in 1970 s – Chloroflourocarbons (refrigerants, aerosols) – one Cl can decompose more than 100, 000 O 3 – Montreal Protocol, 1987: U. N. agreement on ban – up to 10 years for rising CFC gases to reach stratosphere; once in the stratosphere, CFC’s can last up to 50 -100 years


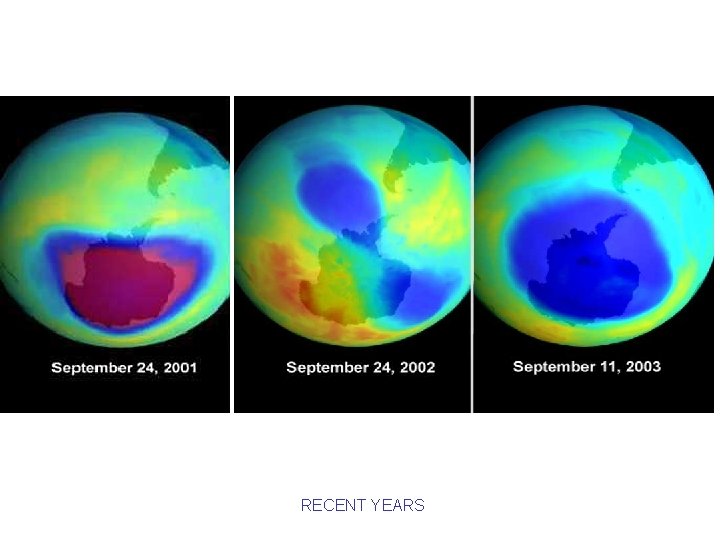
RECENT YEARS

RECENT YEARS

Key Points • Development of Earth’s atmosphere – 4 periods • Vertical structure of the atmosphere – 4 temperature layers – changes in pressure – Aurora borealis / australis – the ozone layer
 For holding one or more test tubes
For holding one or more test tubes Earths early atmosphere contained
Earths early atmosphere contained Whats a natural satellite
Whats a natural satellite Earths interior
Earths interior Arch of constantine frieze
Arch of constantine frieze Whats earths moon called
Whats earths moon called What is luna moon
What is luna moon Earths orbit seasons
Earths orbit seasons Earths biomes
Earths biomes Spring earth tilt
Spring earth tilt What does earths tilt do
What does earths tilt do Whats the thickest layer of the earth
Whats the thickest layer of the earth Earths layer foldable
Earths layer foldable Pangea explanation
Pangea explanation Brown earth soil ireland
Brown earth soil ireland We live in this layer of the atmosphere.
We live in this layer of the atmosphere. Periodic table of elements families
Periodic table of elements families How thick is earths crust
How thick is earths crust Earths boundaries
Earths boundaries Earths roation
Earths roation Earths crust
Earths crust Study of earth's physical features
Study of earth's physical features