Switch to DTG monotherapy DOMONO Study MONCAY Study

- Slides: 6

Switch to DTG monotherapy ‒ DOMONO Study ‒ MONCAY Study ‒ EARLY-SIMPLIFIED Study

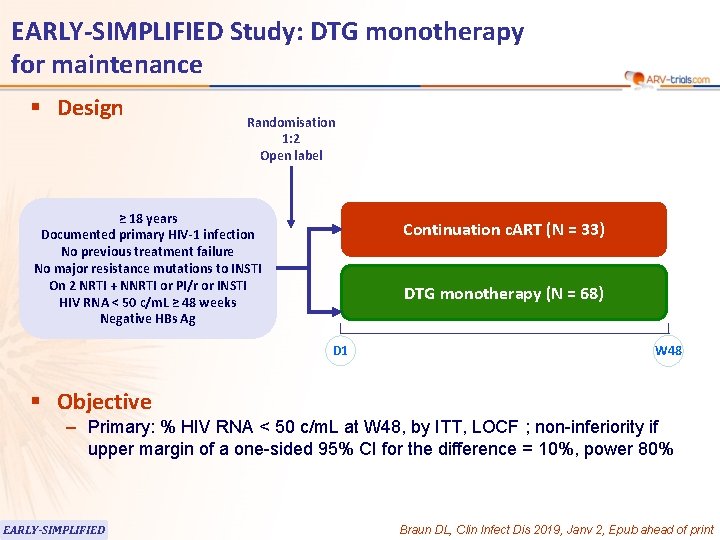
EARLY-SIMPLIFIED Study: DTG monotherapy for maintenance § Design Randomisation 1: 2 Open label ≥ 18 years Documented primary HIV-1 infection No previous treatment failure No major resistance mutations to INSTI On 2 NRTI + NNRTI or PI/r or INSTI HIV RNA < 50 c/m. L ≥ 48 weeks Negative HBs Ag Continuation c. ART (N = 33) DTG monotherapy (N = 68) D 1 W 48 § Objective – Primary: % HIV RNA < 50 c/m. L at W 48, by ITT, LOCF ; non-inferiority if upper margin of a one-sided 95% CI for the difference = 10%, power 80% EARLY-SIMPLIFIED Braun DL, Clin Infect Dis 2019, Janv 2, Epub ahead of print

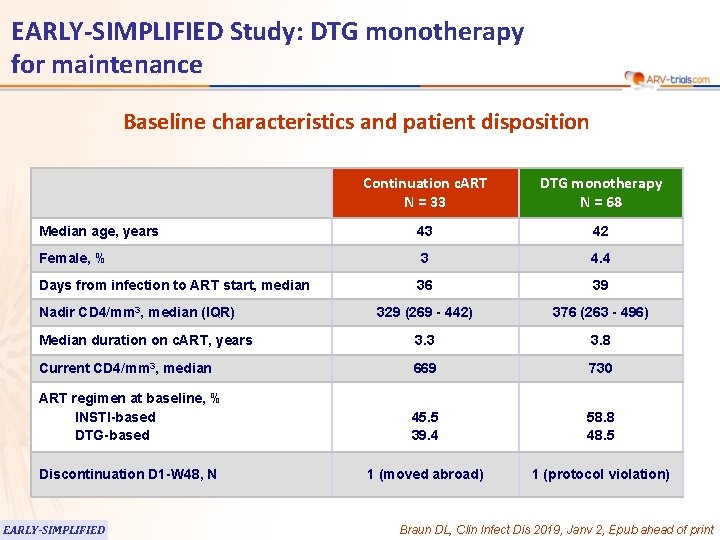
EARLY-SIMPLIFIED Study: DTG monotherapy for maintenance Baseline characteristics and patient disposition Continuation c. ART N = 33 DTG monotherapy N = 68 Median age, years 43 42 Female, % 3 4. 4 Days from infection to ART start, median 36 39 329 (269 - 442) 376 (263 - 496) Median duration on c. ART, years 3. 3 3. 8 Current CD 4/mm 3, median 669 730 ART regimen at baseline, % INSTI-based DTG-based 45. 5 39. 4 58. 8 48. 5 Discontinuation D 1 -W 48, N 1 (moved abroad) 1 (protocol violation) Nadir CD 4/mm 3, median (IQR) EARLY-SIMPLIFIED Braun DL, Clin Infect Dis 2019, Janv 2, Epub ahead of print

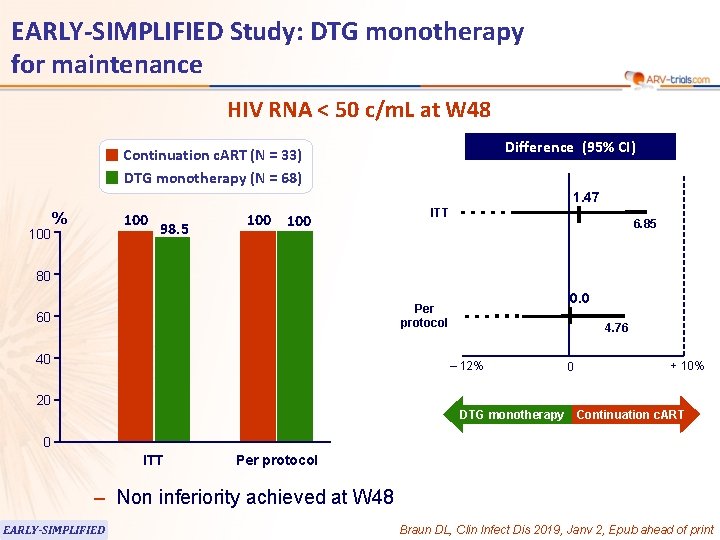
EARLY-SIMPLIFIED Study: DTG monotherapy for maintenance HIV RNA < 50 c/m. L at W 48 Difference (95% CI) Continuation c. ART (N = 33) DTG monotherapy (N = 68) 1. 47 100 % 100 98. 5 100 ITT 6. 85 80 0. 0 Per protocol 60 40 4. 76 ‒ 12% 20 DTG monotherapy 0 + 10% Continuation c. ART 0 ITT Per protocol – Non inferiority achieved at W 48 EARLY-SIMPLIFIED Braun DL, Clin Infect Dis 2019, Janv 2, Epub ahead of print

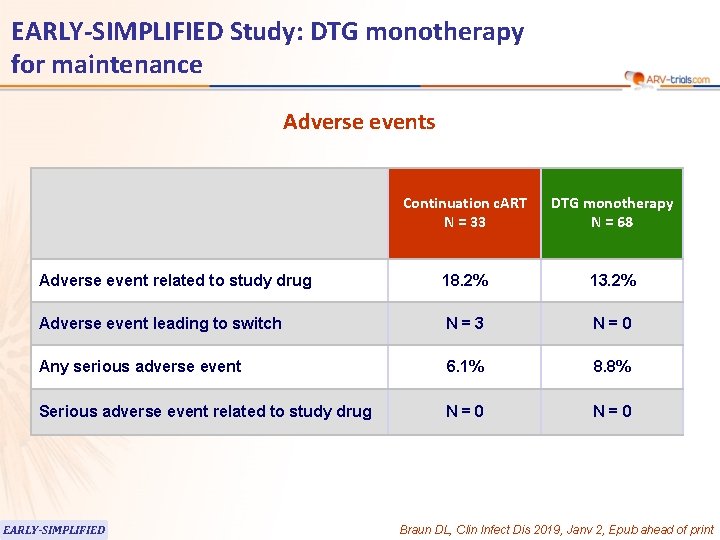
EARLY-SIMPLIFIED Study: DTG monotherapy for maintenance Adverse events Continuation c. ART N = 33 DTG monotherapy N = 68 Adverse event related to study drug 18. 2% 13. 2% Adverse event leading to switch N=3 N=0 Any serious adverse event 6. 1% 8. 8% Serious adverse event related to study drug N=0 EARLY-SIMPLIFIED Braun DL, Clin Infect Dis 2019, Janv 2, Epub ahead of print

EARLY-SIMPLIFIED Study: DTG monotherapy for maintenance § Conclusion – In patients who initiated c. ART < 180 days after the estimated day of a document primary HIV-1 infection and had HIV-1 RNA < 50 c/m. L for more than 48 weeks, DTG monotherapy was non-inferior to c. ART EARLY-SIMPLIFIED Braun DL, Clin Infect Dis 2019, Janv 2, Epub ahead of print