Stoich I If I have 288 quarters how


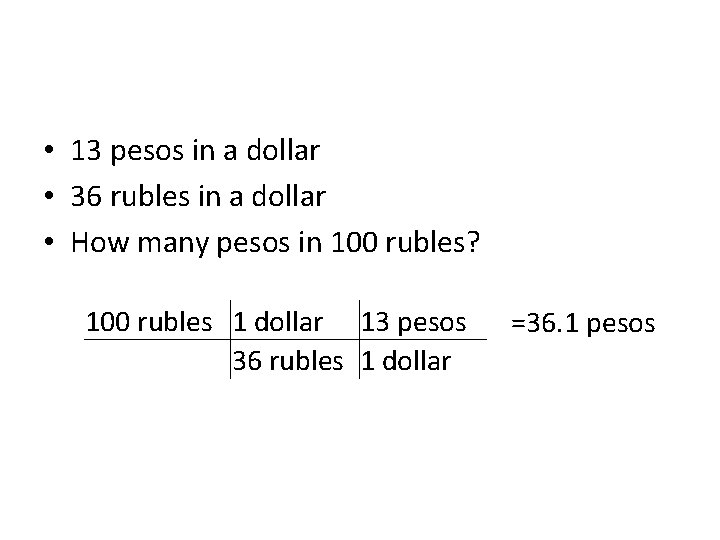




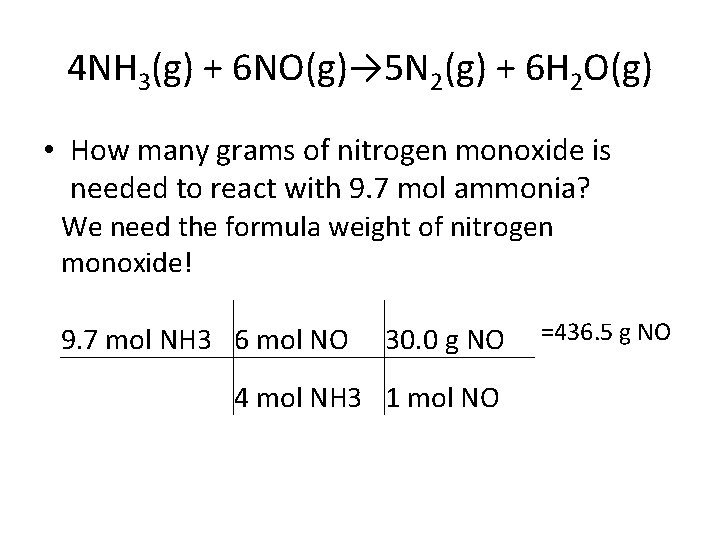

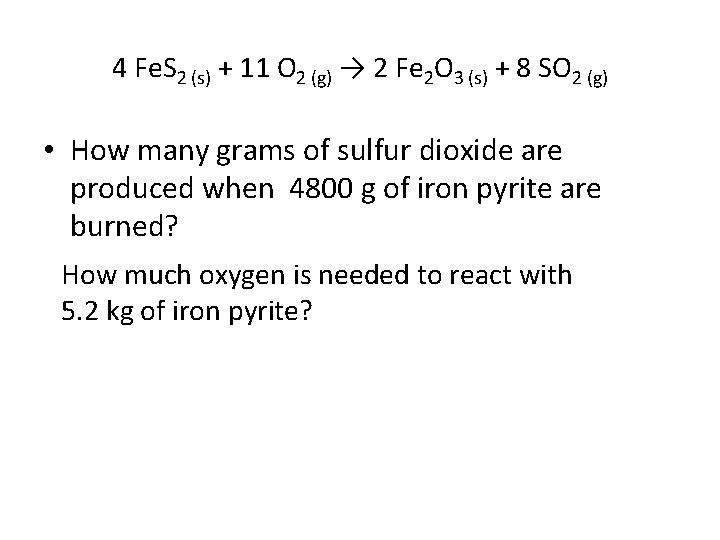

- Slides: 19

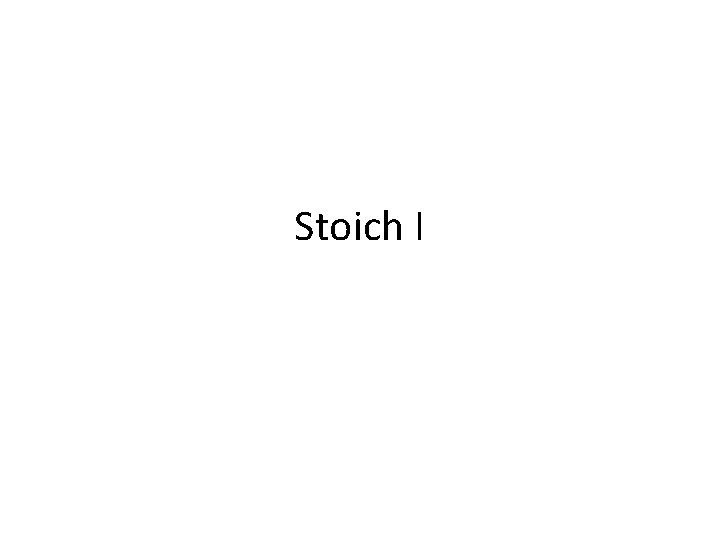
Stoich I

• If I have 288 quarters, how many dimes can I make? • If I have 4 bags of chocolate chips, how many cookies can I make? Both have simple mathematical solutions to solve these and so does stoichiometery!

• Stoichiometry is a method to determine how much of a chemical is produced, or how much is needed, in a reaction. • All it is, is multiplication and division!
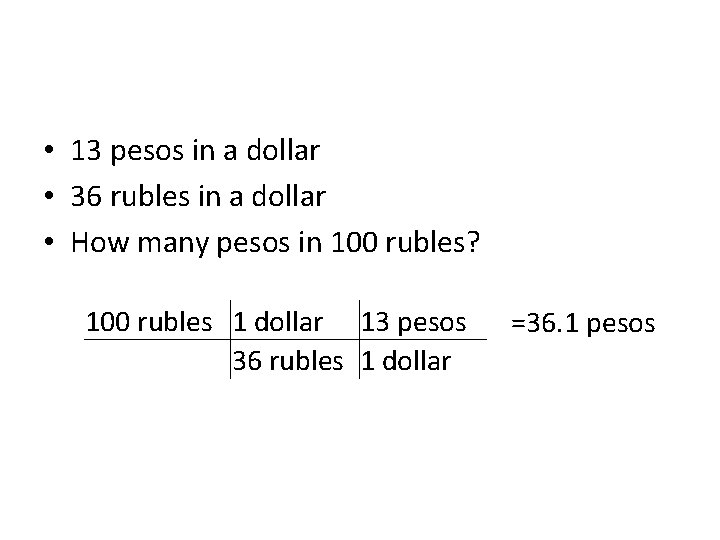
• 13 pesos in a dollar • 36 rubles in a dollar • How many pesos in 100 rubles? 100 rubles 1 dollar 13 pesos 36 rubles 1 dollar =36. 1 pesos

Mole to Mole Conversion • If I have the formula 2 H 2 + O 2 -> 2 H 2 O, how many moles of hydrogen do I need to react with 1 mol of oxygen? 2 mol H 2 • How many moles of hydrogen to I need to react with 5 moles of oxygen? 10 mol H 2

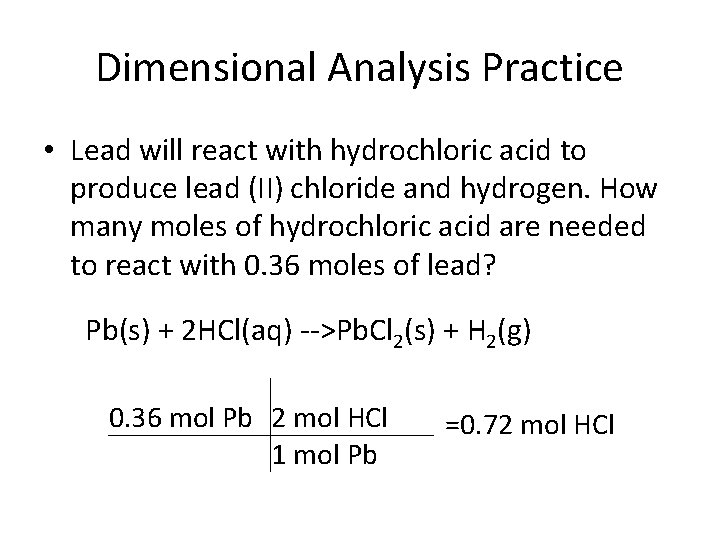
Dimensional Analysis Practice • Lead will react with hydrochloric acid to produce lead (II) chloride and hydrogen. How many moles of hydrochloric acid are needed to react with 0. 36 moles of lead? Pb(s) + 2 HCl(aq) -->Pb. Cl 2(s) + H 2(g) 0. 36 mol Pb 2 mol HCl 1 mol Pb =0. 72 mol HCl

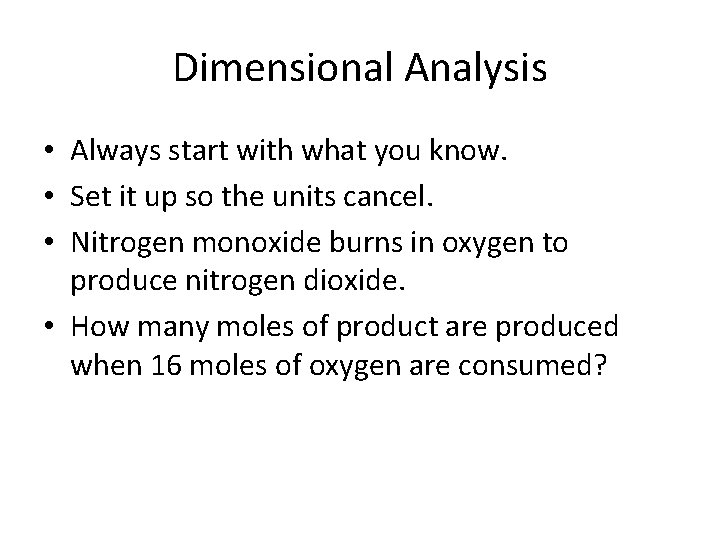
Dimensional Analysis • Always start with what you know. • Set it up so the units cancel. • Nitrogen monoxide burns in oxygen to produce nitrogen dioxide. • How many moles of product are produced when 16 moles of oxygen are consumed?

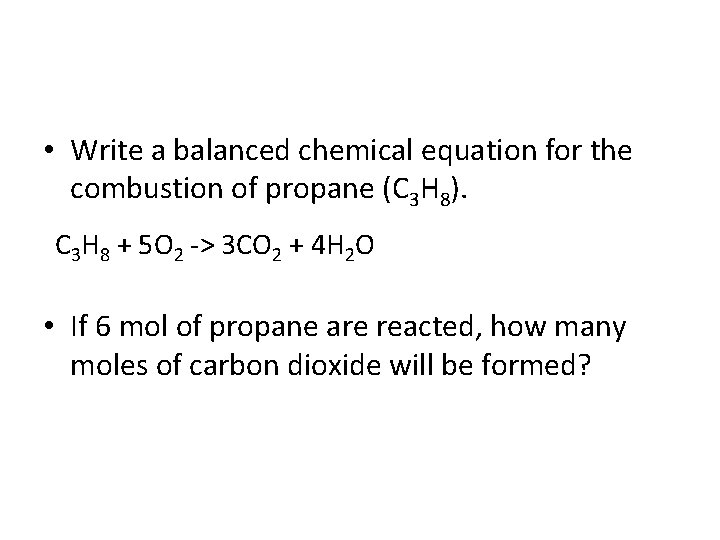
• Write a balanced chemical equation for the combustion of propane (C 3 H 8). C 3 H 8 + 5 O 2 -> 3 CO 2 + 4 H 2 O • If 6 mol of propane are reacted, how many moles of carbon dioxide will be formed?

• If 17 moles of oxygen are used, how many moles of water vapor will be formed? If 24 moles of water vapor are formed, how many moles of propane were reacted? If 33 moles of carbon dioxide were formed, how many moles of water vapor are produced? If 9 moles of oxygen are used, what is the total number of moles of products formed?

Stoich II • This is moles to grams or grams to moles. We need to be able to figure out how much substances weigh! • Remember formula weight? The total weight of all the atoms in a compounds.

Example • A caprese sandwich needs two pieces of bread (100 g), 5 slices of tomatoes (150 g), 4 pieces of mozarella (200 g) and 2 tbs of olive oil (20 g). If we have a package of mozzarella that weighs 440 g, how many sandwiches will it make? 440 g 1 mol tomato 200 g 1 sandwich 1 mol tomato =2. 2 sandwiches

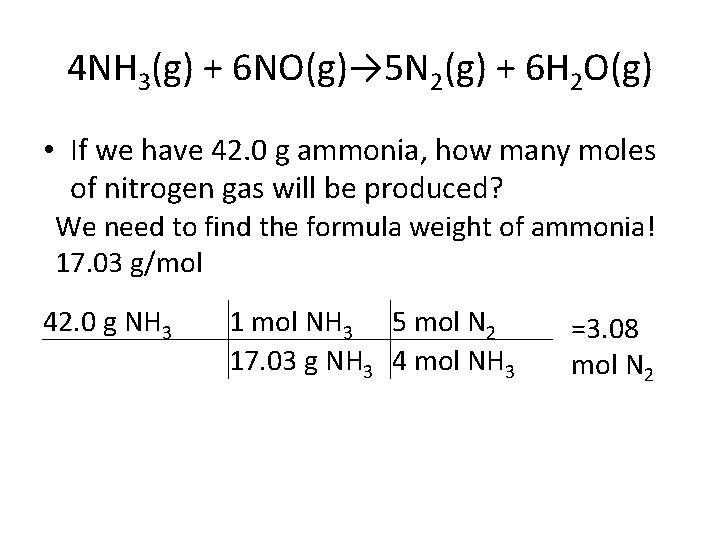
4 NH 3(g) + 6 NO(g)→ 5 N 2(g) + 6 H 2 O(g) • If we have 42. 0 g ammonia, how many moles of nitrogen gas will be produced? We need to find the formula weight of ammonia! 17. 03 g/mol 42. 0 g NH 3 1 mol NH 3 5 mol N 2 17. 03 g NH 3 4 mol NH 3 =3. 08 mol N 2
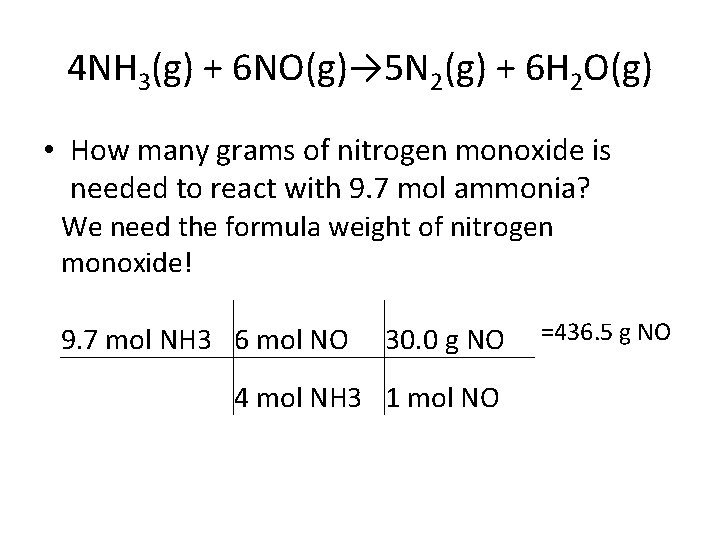
4 NH 3(g) + 6 NO(g)→ 5 N 2(g) + 6 H 2 O(g) • How many grams of nitrogen monoxide is needed to react with 9. 7 mol ammonia? We need the formula weight of nitrogen monoxide! 9. 7 mol NH 3 6 mol NO 30. 0 g NO 4 mol NH 3 1 mol NO =436. 5 g NO

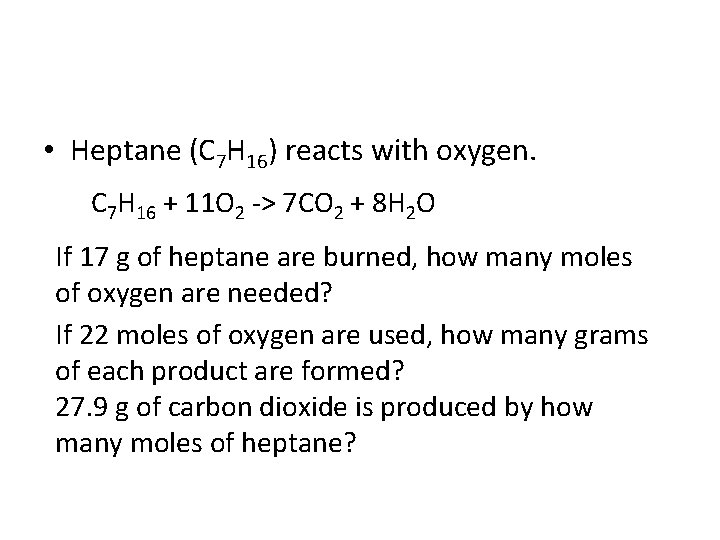
• Heptane (C 7 H 16) reacts with oxygen. C 7 H 16 + 11 O 2 -> 7 CO 2 + 8 H 2 O If 17 g of heptane are burned, how many moles of oxygen are needed? If 22 moles of oxygen are used, how many grams of each product are formed? 27. 9 g of carbon dioxide is produced by how many moles of heptane?

Stoich III • Grams to grams! We need to know the molar mass of both substances we are talking about. • Example. Assume 6 large eggs, 1 cup of cheese (weighs 250 g) and 1 cup of veggies (weighs 400 g) make 2 veggie omelets (each weighs 428 g). If I have 290 g cheese, how many grams of omelets am I making? 290 g cheese 1 c cheese 2 omelets 428 g 250 g cheese 1 c cheese 1 omelet = 992. 6 g omelet

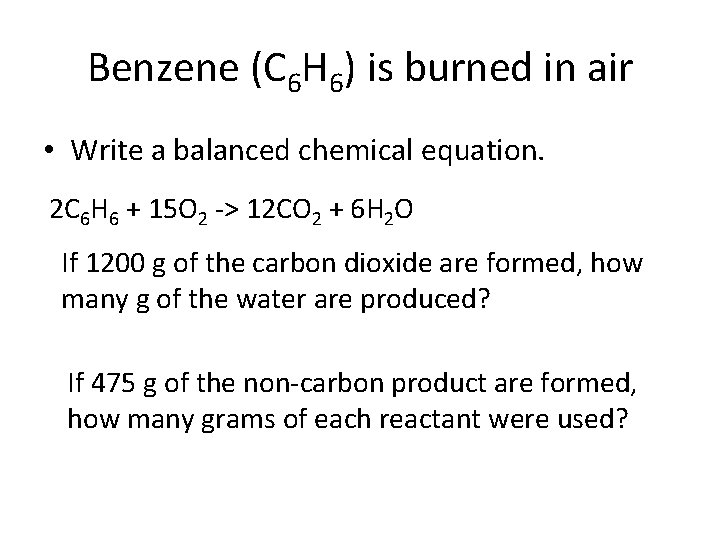
Benzene (C 6 H 6) is burned in air • Write a balanced chemical equation. 2 C 6 H 6 + 15 O 2 -> 12 CO 2 + 6 H 2 O If 1200 g of the carbon dioxide are formed, how many g of the water are produced? If 475 g of the non-carbon product are formed, how many grams of each reactant were used?

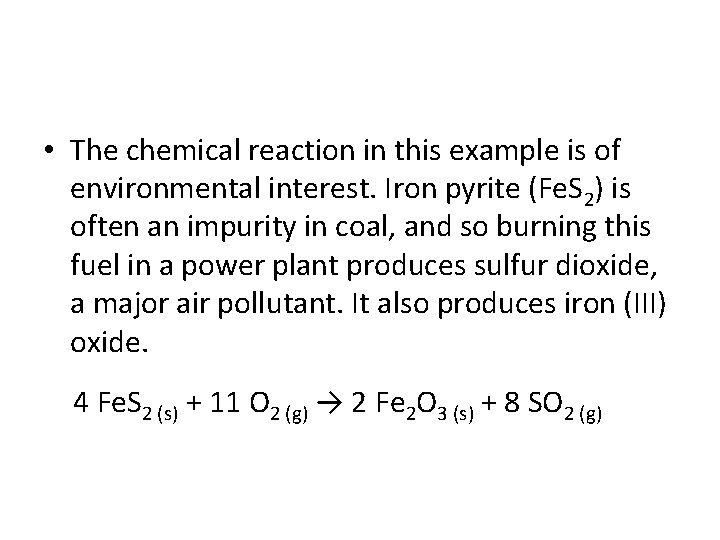
• The chemical reaction in this example is of environmental interest. Iron pyrite (Fe. S 2) is often an impurity in coal, and so burning this fuel in a power plant produces sulfur dioxide, a major air pollutant. It also produces iron (III) oxide. 4 Fe. S 2 (s) + 11 O 2 (g) → 2 Fe 2 O 3 (s) + 8 SO 2 (g)
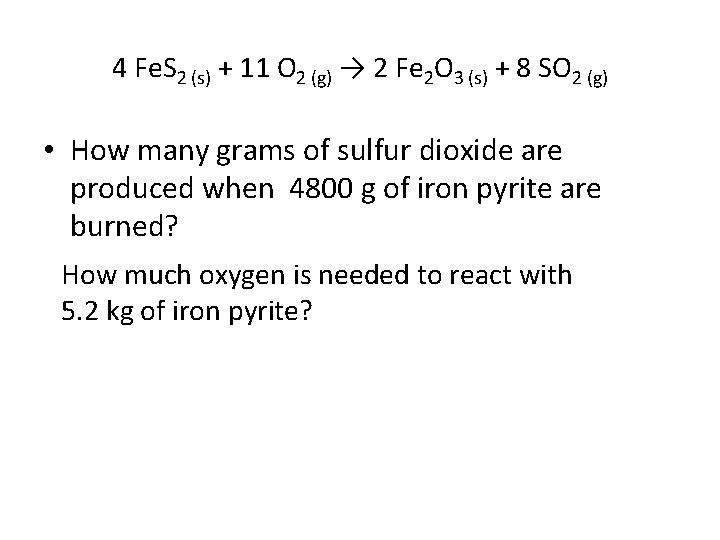
4 Fe. S 2 (s) + 11 O 2 (g) → 2 Fe 2 O 3 (s) + 8 SO 2 (g) • How many grams of sulfur dioxide are produced when 4800 g of iron pyrite are burned? How much oxygen is needed to react with 5. 2 kg of iron pyrite?

• How many grams of magnesium metal are required to react with 35 grams of nitrogen gas to produce magnesium nitride? What mass of lead(II) bromide are formed when a solution containing 2. 45 grams of potassium bromide are mixed with a solution containing 5. 86 grams of lead(II) nitrate? (hint: 2 products are formed!)
 Stoich man
Stoich man Cs 288 njit
Cs 288 njit P 288
P 288 Cpre 288 iowa state
Cpre 288 iowa state Emily dickinson poem 288
Emily dickinson poem 288 Movint client
Movint client Cs 288 njit
Cs 288 njit Pkj 288
Pkj 288 Qqline 288
Qqline 288 Cs 288 njit
Cs 288 njit 4 sections of jerusalem
4 sections of jerusalem Quarter 3 time
Quarter 3 time Quarter past
Quarter past What effect has peeta's comments had on the crowd
What effect has peeta's comments had on the crowd Pronoun and antecedent
Pronoun and antecedent Isaac has 43 nickels and quarters in his wallet
Isaac has 43 nickels and quarters in his wallet Sentio latin conjugation
Sentio latin conjugation The us mint produces quarters that weigh
The us mint produces quarters that weigh Quater past
Quater past M.u.g
M.u.g