STATISTICAL MECHANICS MICROSCOPIC AND MACROSCOPIC SYSTEM MICROSCOPIC AND










- Slides: 16

STATISTICAL MECHANICS

MICROSCOPIC AND MACROSCOPIC SYSTEM

MICROSCOPIC AND MACROSCOPIC SYSTEM • Macroscopic system: system made up of large no of particles. • For describing such a system, macroscopically measurable independent parameters. (temperature, pressure, volume)Eg: Gas in container • For describing microscopic particle, velocity and position of each particles are requred. But it is impractical. We can predict the behaviour of system in terms of macroscopic properties only. Relation connecting microscopic and macroscopic properties gives the idea about microscopic systems

PHASE SPACE

PHASE SPACE • In classical mechanics the dynamical state of system can be described by 3 position co ordinates x , y , z and 3 momentum co-ordinates Px, Py, Pz. • This combined position and momentum space is called PHASE SPACE. • For this we have to imagine 6 D space with 6 mutually perpendicular axis. • If there are N no of particles, Total no of co ordinates-6 N Position co ordinates 3 N and Momentum co ordinates 3 N.

STATISTICAL DISTRIBUTION

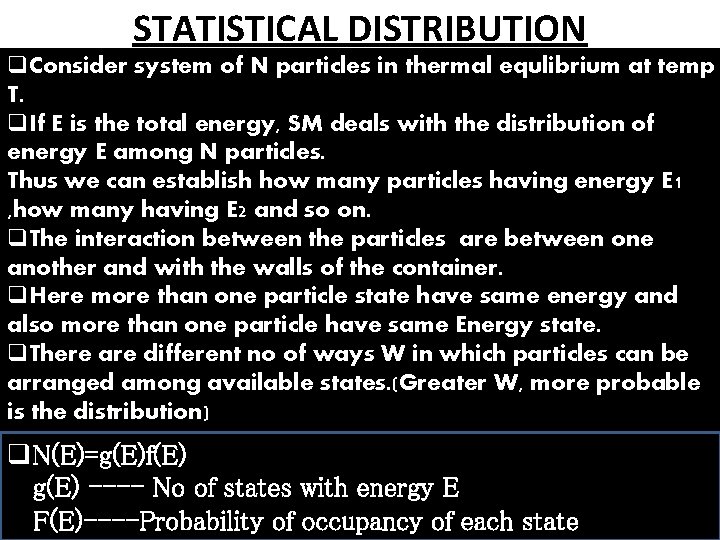
STATISTICAL DISTRIBUTION q. Consider system of N particles in thermal equlibrium at temp T. q. If E is the total energy, SM deals with the distribution of energy E among N particles. Thus we can establish how many particles having energy E 1 , how many having E 2 and so on. q. The interaction between the particles are between one another and with the walls of the container. q. Here more than one particle state have same energy and also more than one particle have same Energy state. q. There are different no of ways W in which particles can be arranged among available states. (Greater W, more probable is the distribution) q. N(E)=g(E)f(E) g(E) ---- No of states with energy E F(E)----Probability of occupancy of each state

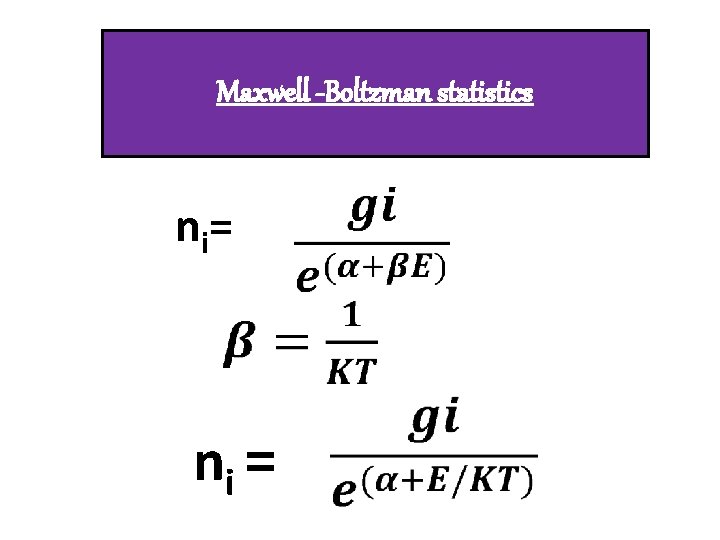
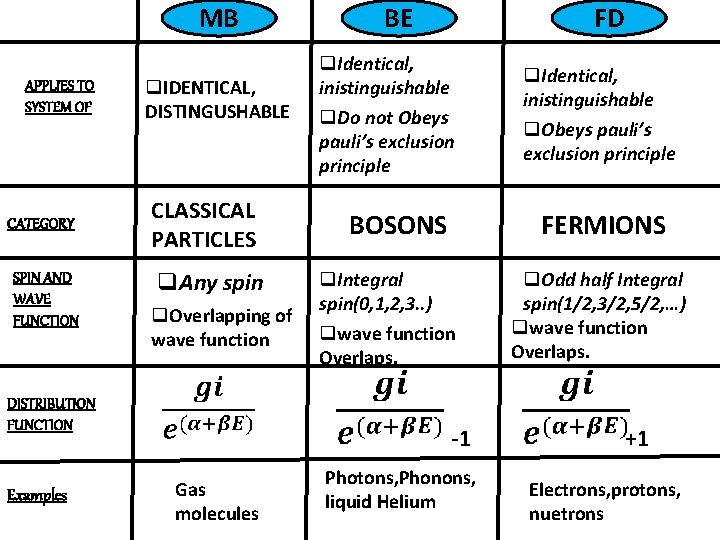
CLASSICAL PARTICLES q. Identical, Distinguishable q. Overlapping of wave function negligible extent. q. Particles having any spin q. Obeys Maxwell- Boltzmann statistics. q. Eg : Gas molecules

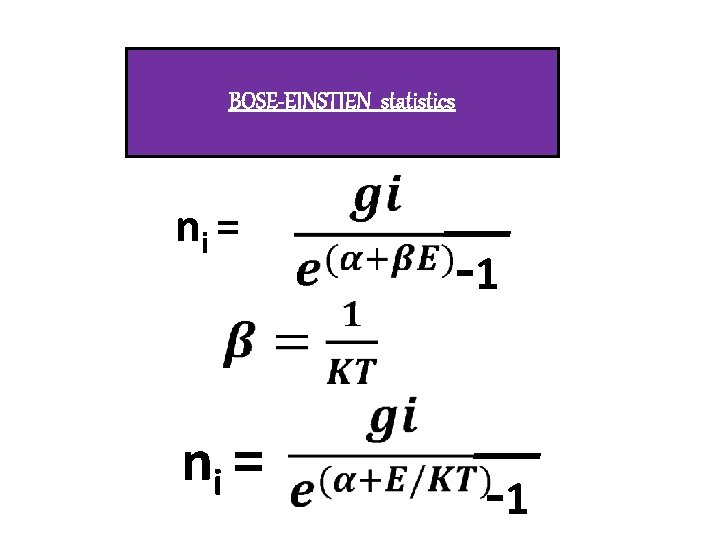
BOSONS q. Identical, inistinguishable qwave function Overlaps. q. Integral spin(0, 1, 2, 3…) q. Do not Obeys pauli’s exclusion principle. q. Obeys Bose –Einstein statistics. q. Eg : photons, particles

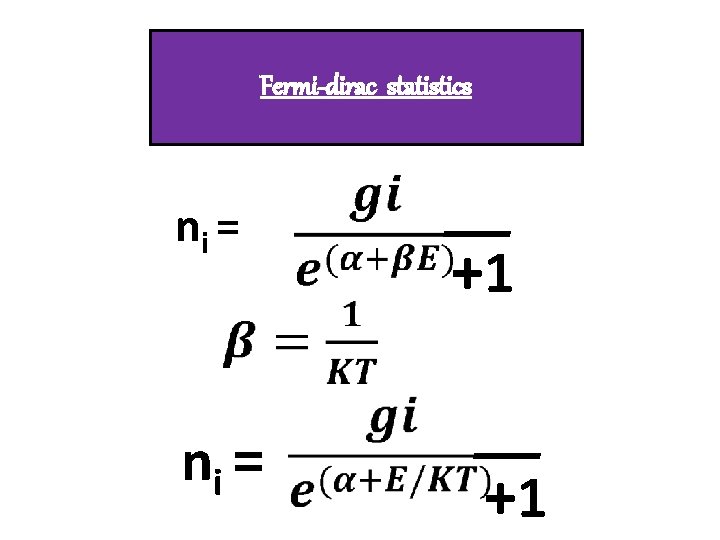
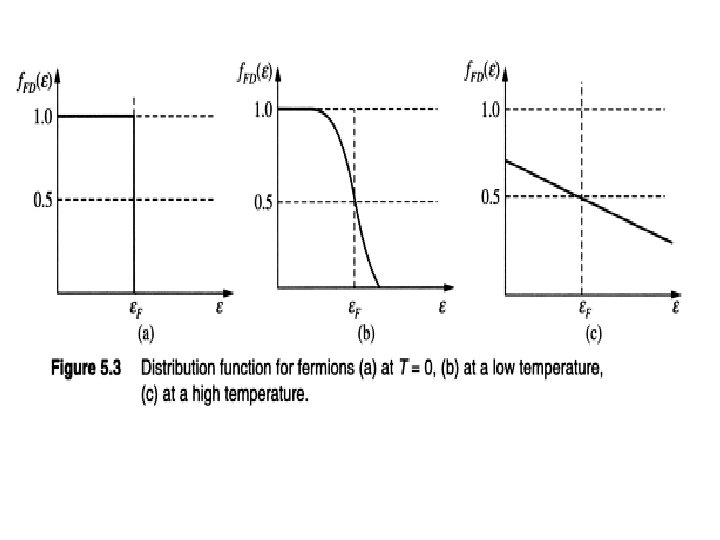
FERMIONS q. Identical, inistinguishable qwave function Overlaps. q. Half Integral spin(1/2) q. Obeys pauli’s exclusion principle. q. Obeys Fermi –Dirac statistics. q. Eg : electrons, protons, nutrons

Maxwell -Boltzman statistics n i= ni =

BOSE-EINSTIEN statistics ni = -1 -1

Fermi-dirac statistics ni = +1 +1


COMPARISON BETWEEN 3 STATISTICS

MB APPLIES TO SYSTEM OF CATEGORY SPIN AND WAVE FUNCTION q. IDENTICAL, DISTINGUSHABLE CLASSICAL PARTICLES q. Any spin q. Overlapping of wave function DISTRIBUTION FUNCTION Examples BE FD q. Identical, inistinguishable q. Do not Obeys pauli’s exclusion principle. BOSONS FERMIONS q. Integral spin(0, 1, 2, 3. . ) qwave function Overlaps. -1 Gas molecules q. Identical, inistinguishable q. Obeys pauli’s exclusion principle. Photons, Phonons, liquid Helium q. Odd half Integral spin(1/2, 3/2, 5/2, …) qwave function Overlaps. +1 Electrons, protons, nuetrons