SKIN SIGNS OF SYSTEMIC DISEASE INTRODUCTION v Skin














- Slides: 24

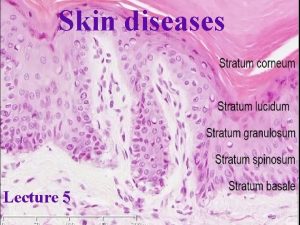
SKIN SIGNS OF SYSTEMIC DISEASE

INTRODUCTION v Skin complaints are some of the commonest reasons patients and parents seek medical attention. Recognizing skin signs provides a unique opportunity for physicians to diagnose systemic diseases early in their course.

OCCULT SPINAL DYSRAPHISM v. Spinal dysraphism (SD) is a group of congenital disorders characterized by absent or incomplete fusion of the midline mesenchymal structures. SD is occult (OSD) when the defect is covered by the overlying skin without exposed neural tissue. OSD can lead to neurological complications if not treated in time: early detection is the key to preventing permanent disabling complications. v. As skin and neural tissue are of common ectodermal origin, developmental defects of these tissues may coexist. The visible skin defects may provide a guide to further

OCCULT SPINAL DYSRAPHISM v. About 75% of OSD cases demonstrate lumbosacral skin changes: - port-wine stain - lipoma - haemangioma - deviated gluteal furrow - dermal sinus - atypical dimple (more than 5 mm in diameter, more than 2. 5 cm from the anus, associated with other lesions) - aplasia cutis congenital - pigmentary abnormalities

OCCULT SPINAL DYSRAPHISM v. Guggisberg et al. suggested categorizing patients with midline lumbosacral skin lesions into three groups (Table based on the associated risk of OSD)


OCCULT SPINAL DYSRAPHISM v. A more recent study from India found 42% of children with 2 or more midline skin lesions had an underlying OSD, but unlike the previous study, also found a high rate of OSD in children with single midline skin lesion such as port wine stain and hemangioma. v. In infants less than 6 months of age : ultrasound (initial imaging study) After 6 months of age: MRI is necessary to rule out OSD If OSD is discovered on imaging studies: follow-up by neurosurgeons

INFANTILE HAEMANGIOMA v. Sporadic benign vascular tumour v 10% of infants v. Often start with a precursor lesion such as a pale or erythematous macule become prominent during the proliferative phase as a bright red lobulated plaque or a nodule. v. Deep haemangiomas present as bluish swelling with normal looking epithelium. Infantile haemangiomas appear within a few weeks after birth, enlarge during a m proliferating phase lasting for the first 6 -9 months of ageand then involute gradually over several years. v. After involution, residual skin atrophy, telangiectasia or excess fibro-fatty tissue may

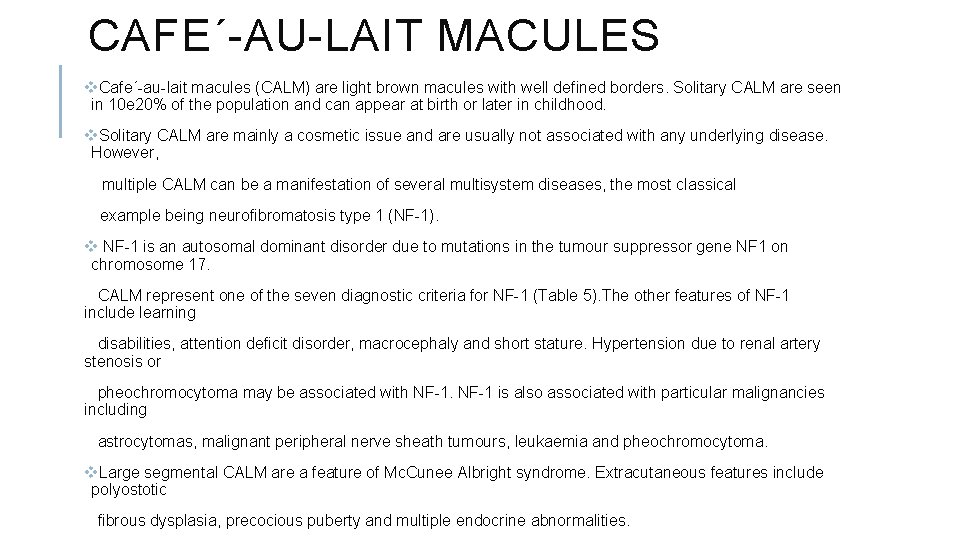
PORT-WINE STAINS Port-wine stains (PWS) are low flow capillary malformations that are usually sporadic and affect about 0. 3% of infants with an equal sex distribution. These PWS are usually present at birth and appear as discrete red vascular patches. PWS are mostly unilateral with a segmental distribution (Figure 2) and a midline cut-off, although bilateral andmultiple PWSinvolvingmultiple dermatomes are seen occasionally. At birth, lesions are pink in colour and with time gradually develop a bluish hue. The lesion grows in proportion to the baby, but with time the surfacemay become more nodular with a thickened, hyperkeratotic surface. PWS can be associated with an underlying arteriovenous anomaly and imaging studies should be considered to rule this out if necessary. Associations with other developmental malformations are summarized below in Table 4.

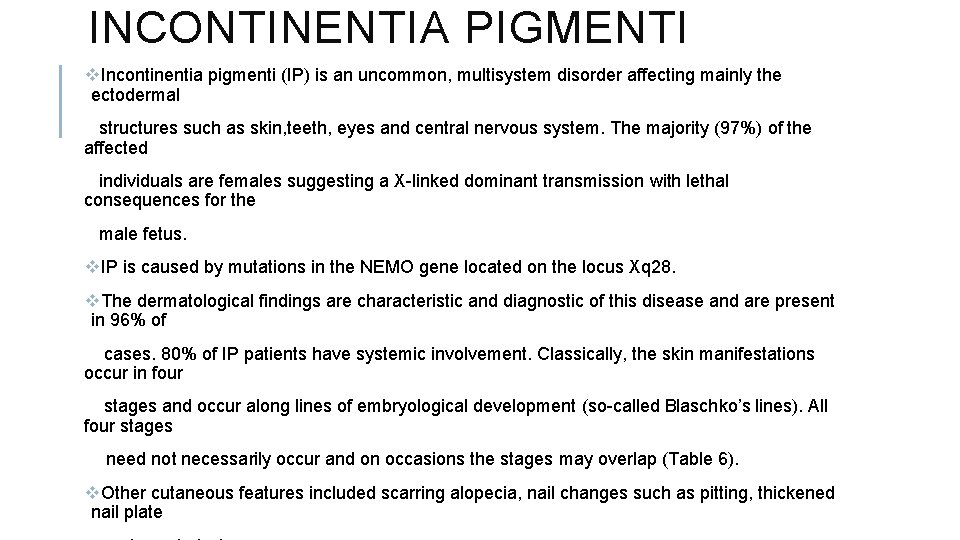
CAFE´-AU-LAIT MACULES v. Cafe´-au-lait macules (CALM) are light brown macules with well defined borders. Solitary CALM are seen in 10 e 20% of the population and can appear at birth or later in childhood. v. Solitary CALM are mainly a cosmetic issue and are usually not associated with any underlying disease. However, multiple CALM can be a manifestation of several multisystem diseases, the most classical example being neurofibromatosis type 1 (NF-1). v NF-1 is an autosomal dominant disorder due to mutations in the tumour suppressor gene NF 1 on chromosome 17. CALM represent one of the seven diagnostic criteria for NF-1 (Table 5). The other features of NF-1 include learning disabilities, attention deficit disorder, macrocephaly and short stature. Hypertension due to renal artery stenosis or pheochromocytoma may be associated with NF-1 is also associated with particular malignancies including astrocytomas, malignant peripheral nerve sheath tumours, leukaemia and pheochromocytoma. v. Large segmental CALM are a feature of Mc. Cunee Albright syndrome. Extracutaneous features include polyostotic fibrous dysplasia, precocious puberty and multiple endocrine abnormalities.

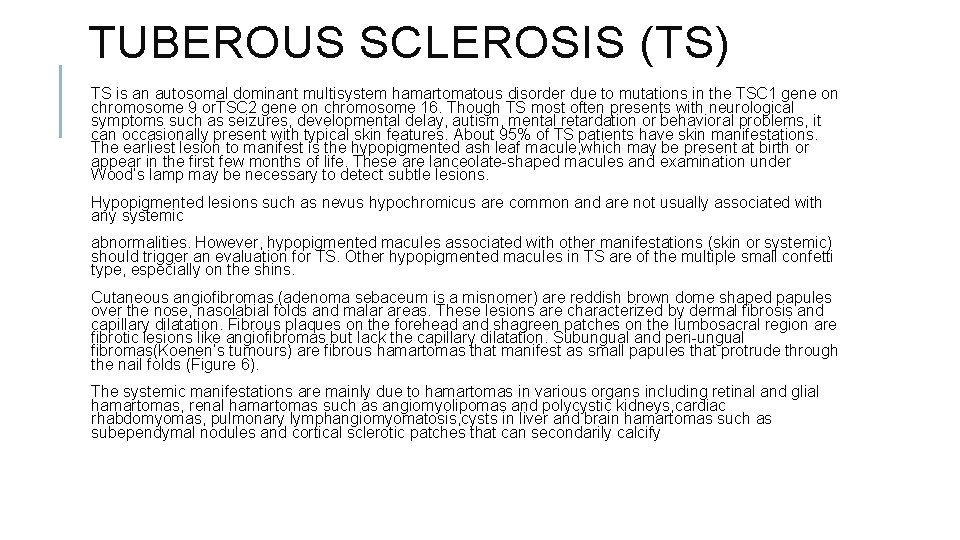
INCONTINENTIA PIGMENTI v. Incontinentia pigmenti (IP) is an uncommon, multisystem disorder affecting mainly the ectodermal structures such as skin, teeth, eyes and central nervous system. The majority (97%) of the affected individuals are females suggesting a X-linked dominant transmission with lethal consequences for the male fetus. v. IP is caused by mutations in the NEMO gene located on the locus Xq 28. v. The dermatological findings are characteristic and diagnostic of this disease and are present in 96% of cases. 80% of IP patients have systemic involvement. Classically, the skin manifestations occur in four stages and occur along lines of embryological development (so-called Blaschko’s lines). All four stages need not necessarily occur and on occasions the stages may overlap (Table 6). v. Other cutaneous features included scarring alopecia, nail changes such as pitting, thickened nail plate

About one-third of IP patients have ocular involvement in the form of strabismus, optic nerve atrophy, nystagmus, micropthalmos, iris hypoplasia, cataracts, uveitis, and abnormalities of the conjunctival pigmentation. Retinal detachment due to progressive fibrovascular retinal changes is the leading cause of blindness in IP. Up to 30% of patients have CNS involvement in the form of seizures, encephalopathy, spasticity, developmental delay, mental retardation, and microcephaly. Infants with IP should be referred to the ophthalmologists forexamination for retinal changes. A complete neurological examination is essential. The role of imaging in IP infants with no obvious neurological deficit is unclear. As there is a good correlation between retinal manifestations and CNS involvement, imaging is indicated in IP patients with retinal abnormalities even if their neurological examination is within normal limits.

TUBEROUS SCLEROSIS (TS) TS is an autosomal dominant multisystem hamartomatous disorder due to mutations in the TSC 1 gene on chromosome 9 or. TSC 2 gene on chromosome 16. Though TS most often presents with neurological symptoms such as seizures, developmental delay, autism, mental retardation or behavioral problems, it can occasionally present with typical skin features. About 95% of TS patients have skin manifestations. The earliest lesion to manifest is the hypopigmented ash leaf macule, which may be present at birth or appear in the first few months of life. These are lanceolate-shaped macules and examination under Wood’s lamp may be necessary to detect subtle lesions. Hypopigmented lesions such as nevus hypochromicus are common and are not usually associated with any systemic abnormalities. However, hypopigmented macules associated with other manifestations (skin or systemic) should trigger an evaluation for TS. Other hypopigmented macules in TS are of the multiple small confetti type, especially on the shins. Cutaneous angiofibromas (adenoma sebaceum is a misnomer) are reddish brown dome shaped papules over the nose, nasolabial folds and malar areas. These lesions are characterized by dermal fibrosis and capillary dilatation. Fibrous plaques on the forehead and shagreen patches on the lumbosacral region are fibrotic lesions like angiofibromas but lack the capillary dilatation. Subungual and peri-ungual fibromas(Koenen’s tumours) are fibrous hamartomas that manifest as small papules that protrude through the nail folds (Figure 6). The systemic manifestations are mainly due to hamartomas in various organs including retinal and glial hamartomas, renal hamartomas such as angiomyolipomas and polycystic kidneys, cardiac rhabdomyomas, pulmonary lymphangiomyomatosis, cysts in liver and brain hamartomas such as subependymal nodules and cortical sclerotic patches that can secondarily calcify

ACRODERMATITIS ENTEROPATHICA Acrodermatitis enteropathica (AE) is caused by deficiency of the trace element zinc, which is found in nuts, shell fish, whole grains and green leafy vegetables. Zinc acts as a catalyst for several metalloenzymes, hence zinc deficiency has wide ranging manifestations. Zinc deficiency may be acquired or inherited and in both instances the manifestations are identical. Inherited zinc deficiency is due to autosomal recessive defect in zinc absorption due to a mutation in the SLC 39 A gene on chromosome 8 q 24. This encodes for the Zip 4 intestinal zinc transporter, responsible for absorbing dietary zinc from the small intestine. Acquired zinc deficiency in children may be caused by anorexia nervosa, breast-feeding, Crohn’s disease, cystic fibrosis, renal insufficiency and dialysis, hepatic or pancreatic insufficiency, HIV infection, intestinal bypass procedures, malabsorption syndromes, prematurity with low zinc storage, restrictive diets (e. g. zinc-deficient vegetarian diets) and total parenteral nutrition. Inherited AE presents shortly after weaning in breast fed infants and within few days to weeks after birth in bottle fed infants. The cutaneous signs of AE are often the first signs of disease and

include erythematous, scaly, crusted, psoriasiform, eczematous, or vesiculobullous eruption around mouth, nose, perineum, ears, eyes (periorificial areas), the extensor surfaces of major joints(elbows, knees, hands, feet) and the scalp. Angular cheilitis is a common feature and involvement of the fingers may cause swelling and paronychia. Secondary infection of the skin lesions with bacteria and Candida albicans is common and may progress to septicemia. The other features of AE are irritability and restlessness, failure to thrive, diarrhea, poor wound healing, alopecia, taste abnormalities, decreased appetite, anaemia, photophobia, neurological disturbances and delayed puberty. Diagnosis of AE can be confirmed with measuring of serum zinc levels. It isessential to measure serum albumin as zinc is bound to albumin and hypoalbuminemia would decrease the zinc levels. The treatment of AE includes addressing any causative factors and other coexistent dietary deficiencies along with oral zinc supplementation. There is 50 mg of elemental zinc in 220 mg of zinc sulfate and therapeutic dosage is 3 mg/kg/d (inherited AE) or 0. 5 e 1. 0 mg/kg/d (acquired AE) of elemental zinc. Restlessness and irritability are the first features to respond, usually within days, even before the zinc levels normalize.

ERYTHROPOIETIC PROTOPORPHYRIA (EPP) v. EPP is an autosomal dominant metabolic disorder due to mutations in the FECH gene on chromosome 18 q 21. 3, which encodes the enzyme ferrochelatase. Ferrochelatase is the final enzyme in the haem synthesis pathway and a deficiency of this enzyme leads to increased protoporphyrins in red blood cells and plasma causing photosensitivity. v. EPP usually presents in infancy and childhood with intense photosensitivity. The photosensitivity usually starts within a few minutes of sun exposure; the history is usually that of a baby crying excessively when outdoors or when exposed to sunlight through windows. Older children present with burning, stinging or prickly sensation on exposure to sunlight. These symptoms can be severe enough to prevent them from going outdoors and they try to relieve the symptoms with cold water application. The diagnosis is often delayed as the health professionals may not take these symptoms seriously: initially there are no clinical signs to go with these intense symptoms. With prolonged sun exposure there will be oedema, erythema, urticarial plaques or occasional purpura seen. With chronic sun exposure, the skin on the face and hands develops waxy thickening and fine superficial depressed scars. v. Normocytic, normochromic anaemia is common, as are porphyrin-containing gall stones. About 5% of EPP patients may develop hepatic failure or cirrhosis and liver transplant may be necessary. Unlike other porphyrias, the urinary porphyrins are not increased as protoporphyrins are not water soluble e the diagnosis is confirmed by demonstration of increased protoporphyrins in the RBCs and plasma. v. Management of EPP is centred on protection from sunlight with appropriate clothing and use of sunscreens. Commercial sunscreens offer protection only against the UVA (320 e 400 nm)and UVB (280 e 320 nm) light with none or very little protection against visible light.

ACANTHOSIS NIGRICANS (AN) AN describes hyperpigmented velvety thickening of skin over the flexures such as axillae, posterior and lateral surfaces of neck (Figure 7), inframammary area, groins and umbilicus. Involvement of the temples and lateral forehead region is particularly common in the Asian population. AN is commonly associated with skin tags. It is often a marker of insulin resistance and its incidence in the paediatric population has gradually risen with increasing incidence of childhood obesity. It usually starts in adolescence, but could start at any age with increasing obesity. “HAIR-AN” syndrome, a syndrome of “Hyper. Androgenism, Insulin resistance and AN” could also be associated with polycystic ovarian syndrome and signs of virilization. Malignancy-associated AN is very rare in children. Treatment of AN is dependent on the cause and children with AN should be considered for referral to a paediatric endocrinologist for evaluation of insulin resistance

SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) SLE is a chronic autoimmune multisystem disorder and can have varied manifestations. About 25% of the patients have the disease onset in the first two decades of life. As emphasized by the revised American College of Rheumatology criteria for the classification (1997), SLE is truly a multisystem disorder and four of the 11 criteria relate to dermatology. About 70 e 80% of SLE patients have skin involvement and this may be in the formofmalar erythema, discoid lupus erythematosus(DLE), photosensitivity, nasal and oral ulceration or scarring alopecia. Raynaud’s phenomenon and vasculitic skin lesions such as petechiae, purpura and ulcers, nail fold telangiectasia, erythema nodosum and lupus panniculitis are also features. A photosensitive eruption may be the first sign of the development of SLE. About half of SLE patients have typical butterfly shaped erythemaover themalar areas and the nose. DLE lesions are erythematous plaques with scaling. They may heal with scarringand hypo- or hyper-pigmentation and are usually seen on the face, ears and scalp. DLE on the scalp may lead to scarring alopecia.

NEONATAL LUPUS ERYTHEMATOSUS (NLE) NLE is a rare condition affecting neonates in which maternal anti-Ro (SSA) and anti-La (SSB) antibodies cross the placenta, bind to the fetal antigens and cause an autoimmune inflammatory reaction. This results in typical cutaneous findings and/or congenital heart block. Mothers of infants with NLE may or may not show symptoms of connective tissue diseases such as Sjogren’s syndrome, SLE or rheumatoid arthritis. Not all infants born to Anti-Ro positive mothers will develop NLE and it has been estimated that the chance of NLE in an infant born to a mother with anti-Ro antibodies is one in 20. The clinical manifestations of NLE are variable: about 50% of infants with NLE will have skin lesions and only 10%of infants with NLE have both cutaneous and cardiac manifestations. The skin lesions develop within the first few days of birth and frequently resemble the annular polycyclic lesions of subacute cutaneous LE. They are erythematous, well-defined plaques which spread peripherally leaving central purpuric annular lesions. The lesions are predominantly located on the scalp and face, though may affect any part of the body. The skin lesions have a predilection for theperiorbital areas which give them an “owl-like” or “spectacle-like” appearance. Exposure to sunlight may precipitate or worsen the lesions. The skin lesions usually heal with clearance of maternal antibodies by 6 e 7 months of age. These lesions do not generally scar, but post-inflammatory pigmentary changes and telengiectasia are common. The morbidity and mortality associated with NLE is mainly due to complete congenital heart block: mortality may be as high as 14% in the first 3 months of life. Other congenital cardiac abnormalities such as patent ductus arteriosus, coarctation of aorta or ventricular septal defects may be present. Infants with NLE may have other manifestations such as hepatitis,

JUVENILE DERMATOMYOSITIS (JDM) JDM is a rare multisystem autoimmune disorder primarily causing proximal muscle weakness due to myositis and typical skin manifestations. A study looking at the clinical features of JDM in 79 children found that all of them had skin lesions and proximal muscleweakness at presentation; the othermanifestations included muscle pain (73%), fever (65%), dysphagia (44%), hoarseness(43%), abdominal pain (37%), arthritis (35%), calcinosis (23%) and melaena (13%). The skin features of JDM are very typical. Heliotrope erythema is a pink or violet erythema of eyelids which may be associated with periorbital oedema. Gottron’s papules are violaceous to erythematous papules over the extensor surfaces of metacarpophalangeal and interphalangeal joints (Figure 9). They can also be seen over the elbows, knees and malleoli. The other dermatological features include ragged cuticles, nail fold telangiectasia, cutaneous ulcers, mucosal ulcers, alopecia and a generalized poikilodermatous rash (atrophy, telangiectasia and variable pigmentation). 40 e 70% of children with JDM develop calcinosis cutis, compared to about 5% of adults with dermatomyositis. This has

JUVENILE DERMATOMYOSITIS (JDM) decreased with aggressive anti-inflammatory treatment, but still can be extensive, troublesome and limit the child’s functional abilities. Myositis, which manifests as a proximal muscle weakness (difficulty in raising arms, rising from a chair, climbing stairs and combing hair) causes significant functional impairment. Muscle pain and tenderness are common and add to the disability. Palatal and pharyngeal muscle involvement can lead to problems with speech and swallowing causing nasal speech, drooling, choking and aspiration. Respiratory muscle myositis can present with acute respiratory failure or with chronic restrictive lung disease. Other systemic manifestations include myocarditis, pericarditis, intestinal mucosal ulceration and nephritis. Diagnosis is by demonstration of the typical rash and proximal muscle weakness. Confirmation of myositis with raised skeletal muscle enzymes AST, LDH and CPK, characteristic EMG findings and muscle biopsy is required. Magnetic resonance imaging of the proximal muscles may demonstrate myositis and help to determine the optimal site of muscle biopsy.

JUVENILE DERMATOMYOSITIS (JDM) Kawasaki disease (KD) KD is an acute febrile multisystem vasculitis mainly affecting infants and children aged less than 5 years. KD has worldwide distribution and affects all ethnic groups. The aetiology is not known. KD has six main features: (1) fever of unknown aetiology of 5 or more days duration e fever is usually of abrupt onset, high grade and 1 e 2 weeks duration; (2) bilateral congestion of ocular conjunctiva with no purulent discharge starts within 2 e 4 days of onset and lasts for less than a week; (3) changes of lips and oral cavity e dryness, redness and fissuring of lips start 3 e 5 days after the onset and can progress to bleeding and hemorrhagic crusting. There is often erythema of the oral and pharyngeal mucosa and tongue (“strawberry tongue”); (4) Acute nonpurulent cervical lymphadenopathy; (5) Polymorphous exanthem e cutaneous lesions are often polymorphic and can be a combination of macular, maculopapular, urticarial plaques and erythema multiforme-like lesions. Vesicles and crusting are usually not seen. The skin lesions start 1 e 5 days after the onset of fever and last for less than a week; (6) Acral changes e these changes start 2 e 5 days after the onset of fever and manifest as diffuse erythema of the palms and soles with oedema. After 10 e 15 days the oedema subsides and there is desquamation of the finger tips, sometimes progressing to the palms and soles. Systemic manifestations include diarrhea, small bowel obstruction, ileus, hepatitis, pancreatitis, hydrops of gallbladder, arthralgia/arthritis, anterior uveitis and aseptic meningitis. Laboratory abnormalities include leucocytosis, thrombocytosis and raised CRP and ESR. The most important of all systemic manifestations is the cardiovascular involvement with about 25% of all untreated KD patients later developing coronary artery dilatation or aneurysms. These changes can start as early as the 10 th day of illness and in half of these patients the coronary artery changes have regressed in 6 e 18 months. As there is no diagnostic test for KD, the diagnosis depends on the six main features. If five or six features are present, KD can be diagnosed after excluding other causes. In patients who have coronary artery aneurysms, KD can be diagnosed with four or less of the six main features.

LANGERHANS’ CELL HISTIOCYTOSIS (LCH) Langerhans’ cells are the main antigen-presenting cells of the epidermis. LCH is characterized by the proliferation of these Langerhans’ cells. Though clonality of these Langerhans’ cell proliferation has been demonstrated, there is still no consensus as to whether this is a malignant or a reactive process. Children with LCH may present with a rash. Some present with diabetes insipidus. The rash is usually in the form multiple papules and macules, often with purpura involving the seborrhoeic areas(scalp, retro-auricular areas, face, groins and axillae). The flexural areas are prone to fissuring and ulceration. Nail dystrophy is common. Rarely, hypopigmented macules and papules (especially in darker skin) and nodules can occur with LCH. Systemic involvement may manifest with lymphadenopathy, hepatomegaly, bone marrow involvement, lytic bone lesions, parapituitary infiltration causing diabetes insipidus or exophthalmos due to orbital involvement. Diagnosis is confirmed by biopsying one or more involved organs to demonstrate the typical. Langerhan’s cell e a mononuclear cell with a foamy cytoplasm and a kidney-shaped pale nucleus with immunohistochemistry showing S-100 and CD 1 a positivity. Further investigations to assess the extent of other organ involvement are essential

ERYTHRODERMA Erythroderma describes inflammation of the whole or almost the entire skin surface manifesting as generalized erythema with or without scaling. It may be associated with alopecia, nail changesand skin infiltration. Systemic examination may reveal lymphadenopathy, hepatosplenomegaly and oedema. The predominant cause varies with age and in adults a pre-existing skin disease(such as psoriasis, atopic eczema, pityriasis rubra pilaris, seborroheic dermatitis) is the commonest. The aetiology of erythroderma in children varies: in one series of 17 children, drug reactions (29%), genodermatoses, psoriasis, and staphylococcal scalded skin syndrome (18% each) were the common causes. Determining the precise aetiology may require extended follow-up in many instances. In another series of 51 patients with neonatal and infantile erythroderma, there was an average of 11 months delay before diagnosis. Ultimately, immunodeficiency disorders such as Omenn syndrome, severe combined immunodeficiency disease, Wiskotte. Aldrich syndrome and Ig. A deficiency were diagnosed in
 All the signs for driving
All the signs for driving Bharathi viswanathan
Bharathi viswanathan Natural signs in semantics
Natural signs in semantics The color of a recreation area sign is ______________.
The color of a recreation area sign is ______________. The diamond shape is used exclusively for _____ signs
The diamond shape is used exclusively for _____ signs Carotenemia
Carotenemia Tree skin disease
Tree skin disease Skin is the largest organ
Skin is the largest organ Thin skin vs thick skin
Thin skin vs thick skin Describe the differences between alipidic and oily skin.
Describe the differences between alipidic and oily skin. How much kontos per gallon
How much kontos per gallon Mycoses
Mycoses Local or systemic
Local or systemic Systemic effects of immobility
Systemic effects of immobility Systemic circulation flow chart
Systemic circulation flow chart Systemic game design
Systemic game design Normal pulmonary vascular resistance
Normal pulmonary vascular resistance Identify sources of systemic fluoride
Identify sources of systemic fluoride Overview of the major systemic arteries
Overview of the major systemic arteries Plants are sessile
Plants are sessile Systemic pathology mcqs
Systemic pathology mcqs Systemic acquired resistance in plants
Systemic acquired resistance in plants Absolute contraindication for extraction of teeth
Absolute contraindication for extraction of teeth Bp = co x svr
Bp = co x svr Human diseases a systemic approach
Human diseases a systemic approach