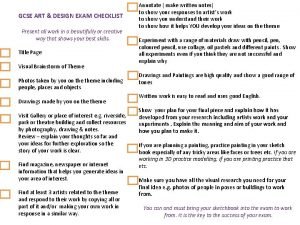
Session 6 Chemistry SUMMARY OF ASSESSMENT GCSE CHEMISTRY







- Slides: 17

Session 6 Chemistry

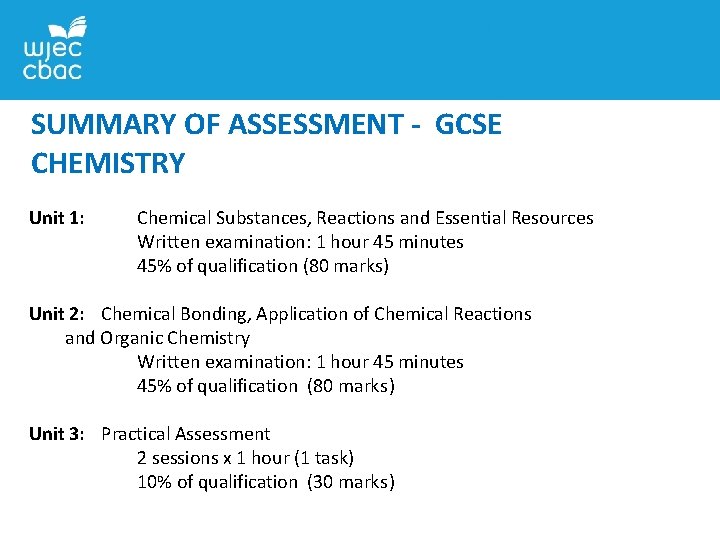
SUMMARY OF ASSESSMENT - GCSE CHEMISTRY Unit 1: Chemical Substances, Reactions and Essential Resources Written examination: 1 hour 45 minutes 45% of qualification (80 marks) Unit 2: Chemical Bonding, Application of Chemical Reactions and Organic Chemistry Written examination: 1 hour 45 minutes 45% of qualification (80 marks) Unit 3: Practical Assessment 2 sessions x 1 hour (1 task) 10% of qualification (30 marks)

SUMMARY OF ASSESSMENT – GCSE SCIENCE (DOUBLE AWARD) Unit 2: (Double Award) CHEMISTRY 1 Written examination: 1 hour 15 minutes 15% of qualification (60 marks) Unit 5: (Double Award) CHEMISTRY 2 Written examination: 1 hour 15 minutes 15% of qualification (60 marks) Unit 7: Practical Assessment 4 sessions x 1 hour (2 tasks) 10% of qualification (60 marks)

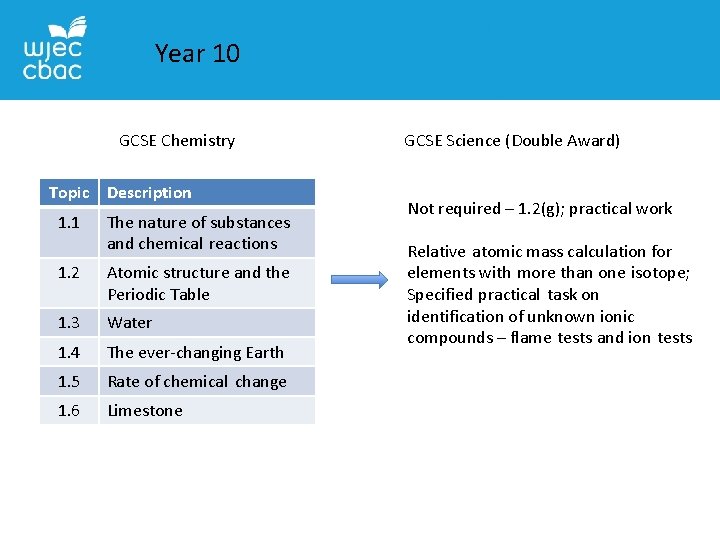
Year 10 GCSE Chemistry Topic Description 1. 1 The nature of substances and chemical reactions 1. 2 Atomic structure and the Periodic Table 1. 3 Water 1. 4 The ever-changing Earth 1. 5 Rate of chemical change 1. 6 Limestone GCSE Science (Double Award) Not required – 1. 2(g); practical work Relative atomic mass calculation for elements with more than one isotope; Specified practical task on identification of unknown ionic compounds – flame tests and ion tests

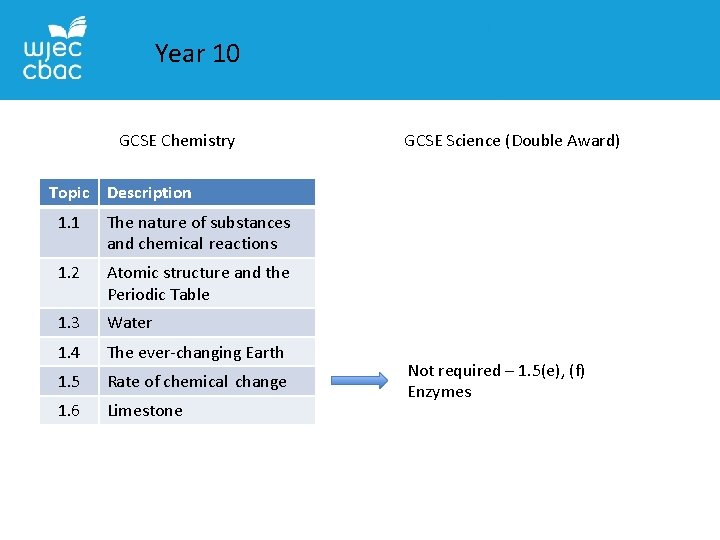
Year 10 GCSE Chemistry GCSE Science (Double Award) Topic Description 1. 1 The nature of substances and chemical reactions 1. 2 Atomic structure and the Periodic Table 1. 3 Water 1. 4 The ever-changing Earth 1. 5 Rate of chemical change 1. 6 Limestone Not required – 1. 5(e), (f) Enzymes

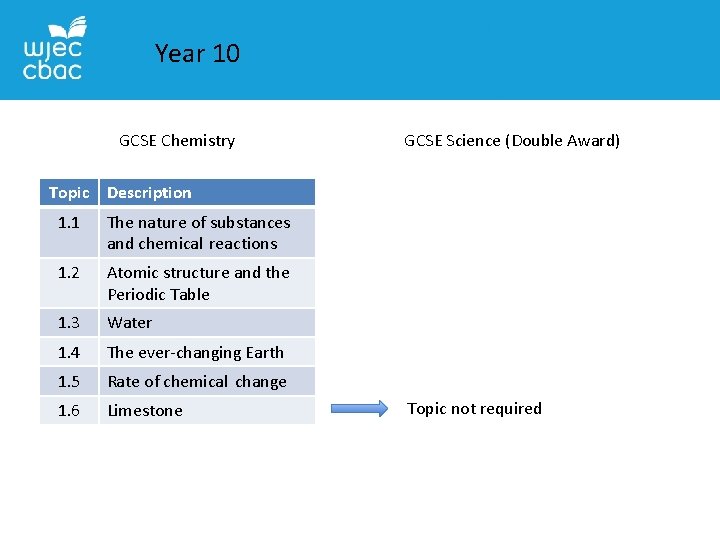
Year 10 GCSE Chemistry GCSE Science (Double Award) Topic Description 1. 1 The nature of substances and chemical reactions 1. 2 Atomic structure and the Periodic Table 1. 3 Water 1. 4 The ever-changing Earth 1. 5 Rate of chemical change 1. 6 Limestone Topic not required

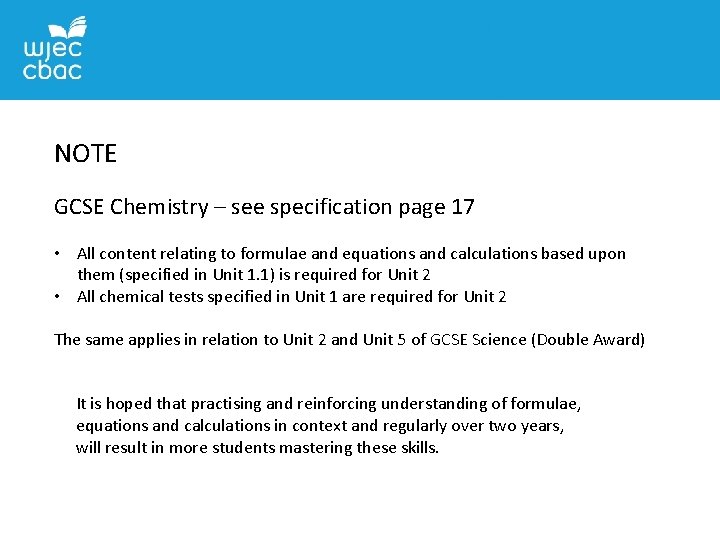
NOTE GCSE Chemistry – see specification page 17 • All content relating to formulae and equations and calculations based upon them (specified in Unit 1. 1) is required for Unit 2 • All chemical tests specified in Unit 1 are required for Unit 2 The same applies in relation to Unit 2 and Unit 5 of GCSE Science (Double Award) It is hoped that practising and reinforcing understanding of formulae, equations and calculations in context and regularly over two years, will result in more students mastering these skills.

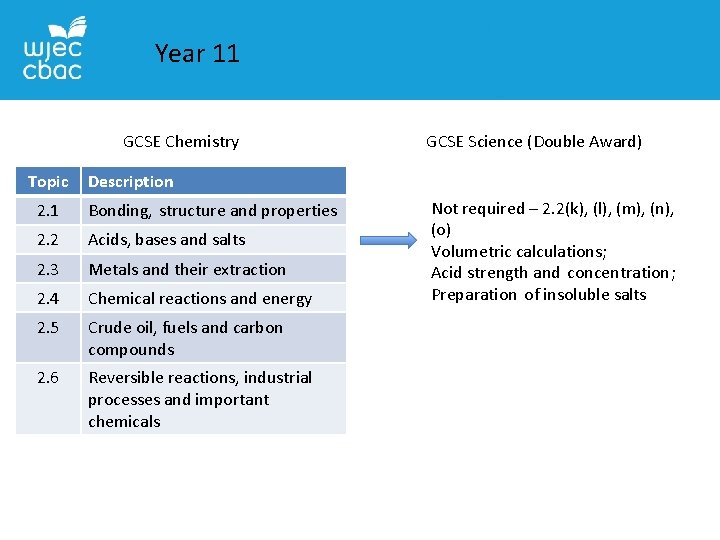
Year 11 GCSE Chemistry Topic GCSE Science (Double Award) Description 2. 1 Bonding, structure and properties 2. 2 Acids, bases and salts 2. 3 Metals and their extraction 2. 4 Chemical reactions and energy 2. 5 Crude oil, fuels and carbon compounds 2. 6 Reversible reactions, industrial processes and important chemicals Not required – 2. 2(k), (l), (m), (n), (o) Volumetric calculations; Acid strength and concentration; Preparation of insoluble salts

Year 11 GCSE Chemistry Topic GCSE Science (Double Award) Description 2. 1 Bonding, structure and properties 2. 2 Acids, bases and salts 2. 3 Metals and their extraction 2. 4 Chemical reactions and energy 2. 5 Crude oil, fuels and carbon compounds 2. 6 Reversible reactions, industrial processes and important chemicals Not required – 2. 3(k), (m), (n), (o), (p) Precipitation of metal hydroxides; Electrolysis of aqueous solutions; Specified practical task on electrolysis and electroplating

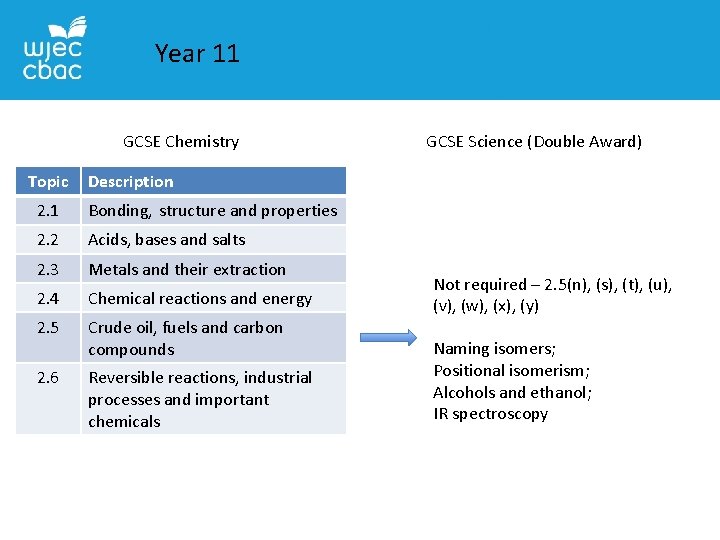
Year 11 GCSE Chemistry Topic GCSE Science (Double Award) Description 2. 1 Bonding, structure and properties 2. 2 Acids, bases and salts 2. 3 Metals and their extraction 2. 4 Chemical reactions and energy 2. 5 Crude oil, fuels and carbon compounds 2. 6 Reversible reactions, industrial processes and important chemicals Not required – 2. 5(n), (s), (t), (u), (v), (w), (x), (y) Naming isomers; Positional isomerism; Alcohols and ethanol; IR spectroscopy

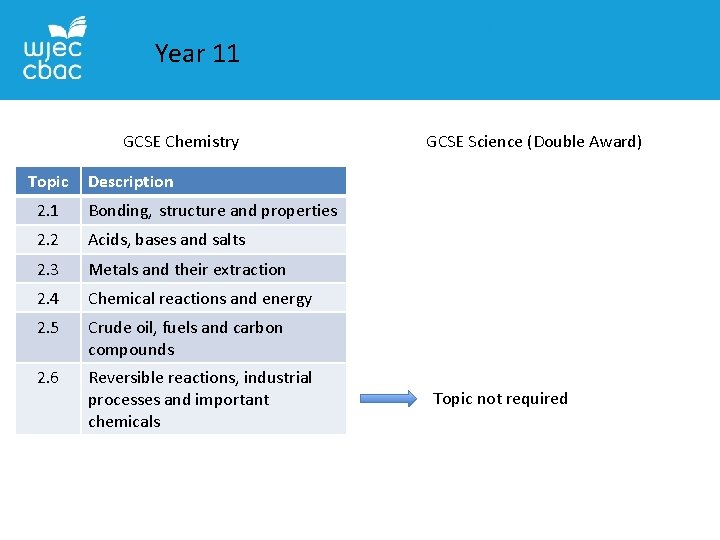
Year 11 GCSE Chemistry Topic GCSE Science (Double Award) Description 2. 1 Bonding, structure and properties 2. 2 Acids, bases and salts 2. 3 Metals and their extraction 2. 4 Chemical reactions and energy 2. 5 Crude oil, fuels and carbon compounds 2. 6 Reversible reactions, industrial processes and important chemicals Topic not required

Specified Practical Work Year 10 Units • Identification of unknown ionic compounds using flame tests and chemical tests for ions (GCSE Chemistry only) • Determination of the amount of hardness in water using soap solution • Investigation of the factors that affect the rate of a reaction using a gas collection method • Investigation of the factors that affect the rate of the reaction between dilute hydrochloric acid and sodium thiosulfate • Investigation of thermal stabilities of calcium carbonate, copper(II) carbonate and sodium carbonate (GCSE Chemistry only)

Specified Practical Work Year 11 Units • Preparation of crystals of a soluble salt from an insoluble base or carbonate • Titration of a strong acid against a strong base using an indicator • Determination of relative reactivities of metals through displacement reactions • Investigation into electrolysis of aqueous solutions and electroplating (GCSE Chemistry only) • Determination of the amount of energy released by a fuel

Relative atomic mass – not mass number No instruction to use ion table or Periodic Table within questions

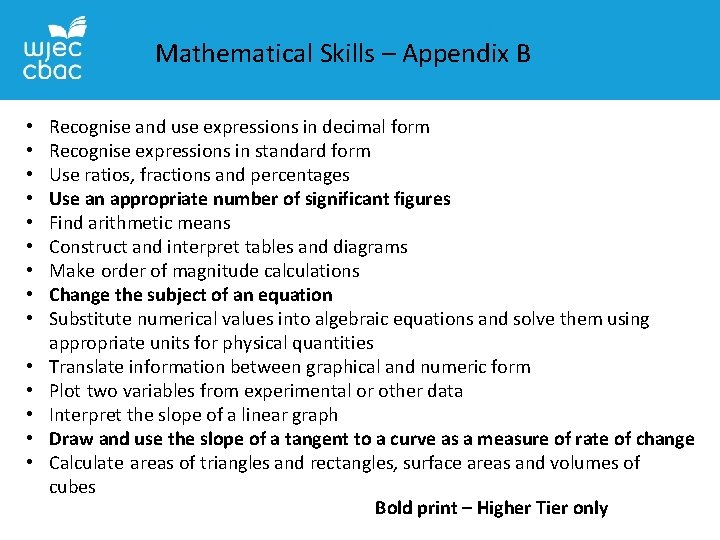
Mathematical Skills – Appendix B • • • • Recognise and use expressions in decimal form Recognise expressions in standard form Use ratios, fractions and percentages Use an appropriate number of significant figures Find arithmetic means Construct and interpret tables and diagrams Make order of magnitude calculations Change the subject of an equation Substitute numerical values into algebraic equations and solve them using appropriate units for physical quantities Translate information between graphical and numeric form Plot two variables from experimental or other data Interpret the slope of a linear graph Draw and use the slope of a tangent to a curve as a measure of rate of change Calculate areas of triangles and rectangles, surface areas and volumes of cubes Bold print – Higher Tier only

Chemistry Resources • Guidance on specified practical tasks • Digital resources • Guidance for Teaching http: //www. wjec. co. uk/qualifications/scienc e/gcse/chemistry-gcse-2016 /

Any Questions?
 Word 2016 session 2 post assessment
Word 2016 session 2 post assessment Session 4 post assessment
Session 4 post assessment Edexcel art gcse past papers
Edexcel art gcse past papers Gcse art annotation format
Gcse art annotation format Ocr gcse pe nea
Ocr gcse pe nea Pixl knowit gcse chemistry chemical changes
Pixl knowit gcse chemistry chemical changes Aqa chemistry gcse required practicals
Aqa chemistry gcse required practicals Principles of portfolio assessment
Principles of portfolio assessment Define dynamic assessment
Define dynamic assessment Portfolio assessment matches assessment to teaching
Portfolio assessment matches assessment to teaching Chapter 11 stoichiometry study guide answer key
Chapter 11 stoichiometry study guide answer key Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Situational leadership grid
Situational leadership grid Cao qualifications & assessment summary
Cao qualifications & assessment summary Session 0 windows
Session 0 windows Welcome to new session 2020-21
Welcome to new session 2020-21 Session
Session