Section 4 3 VSEPR Valence Shell Electron Pair






- Slides: 11

Section 4. 3

� VSEPR (Valence Shell Electron Pair Repulsion) �valence electrons stay as far apart as possible to minimize repulsion � Tells us the geometry of the molecule � When looking at a molecule we look specifically at the central atom (the one that has the most bonding electrons) to determine the geometry

� Only the valence shell electrons of the central atom(s) are important for molecular shape � Valence shell electrons are paired or will be paired in a molecule or polyatomic ion � Bonded pairs or electrons and lone pairs of electrons are treated approximately equal � Valence shell electron pairs repel each other electrostatically (“like repels like”) � The molecular shape is determined by the positions of the electron pairs when they are a maximum distance apart.

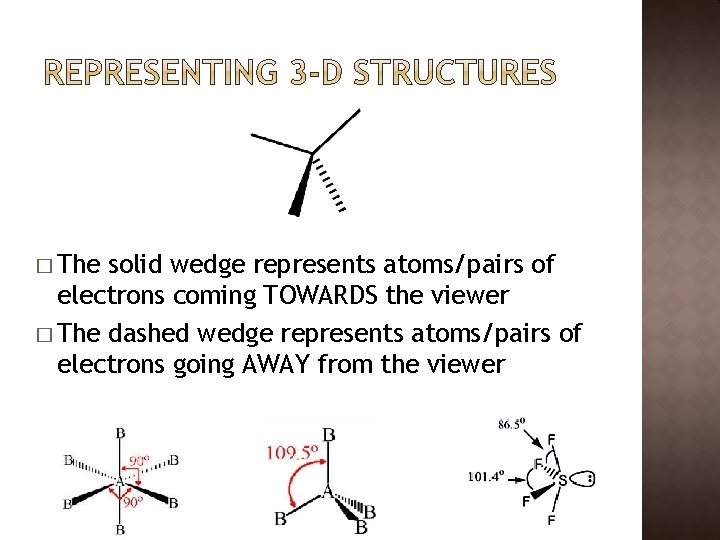
� The solid wedge represents atoms/pairs of electrons coming TOWARDS the viewer � The dashed wedge represents atoms/pairs of electrons going AWAY from the viewer

� First draw Lewis structures of the molecule, including the electron pairs around the central atom � Count the total number of bonding pairs and lone pairs around the central atom � Refer to table 1 on pg. 245 or Appendix C 3 to predict the shape

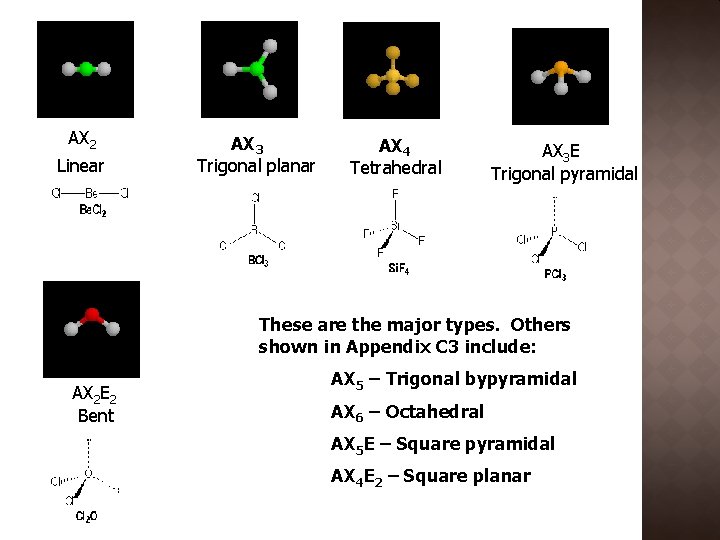
AX 2 Linear AX 3 Trigonal planar AX 4 Tetrahedral AX 3 E Trigonal pyramidal These are the major types. Others shown in Appendix C 3 include: AX 2 E 2 Bent AX 5 – Trigonal bypyramidal AX 6 – Octahedral AX 5 E – Square pyramidal AX 4 E 2 – Square planar


� What are the shapes of the following molecules: nitrogen trihydride dihydrogen sulfide methane boron trichloride

� Secrets �Sulfur we’ve been keeping: can have several different valence configurations: It is actually found in nature as S 8 Can have 2, 4, 6 unpaired electrons �Phosphorous Can have 3, 4, 5 unpaired electrons �Basically the larger the atom the more electrons it can have. �And some noble gases form compounds! � When in doubt use the method of adding up valence electrons from yesterday to determine the # of l. p. of e- on the central atom.

phosphorous pentafluoride bromine pentachloride xenon tetrafluoride sulfur hexafluoride

� Iodine dichloride ion (ICl 2 -) � Chlorine trifluoride (Cl. F 3) � Sulfur tetrafluoride (SF 4) � When you are done complete the worksheet. (we are working on this tomorrow too!)