Molecule Shapes VSEPR Theory Valence Shell Electron Pair










- Slides: 25

Molecule Shapes

• VSEPR Theory: Valence Shell Electron Pair Repulsion Theory – Valence Shell – electrons in the highest energy level – Electron Pair – Repulsion – pushing away • Pairs of electrons want to be as far from each other as possible in a molecule

• Non-bonding electrons: pairs of electrons that are not part of a bond. • Area of high electron density: an “end” of molecule or a non-bonding electron pair.

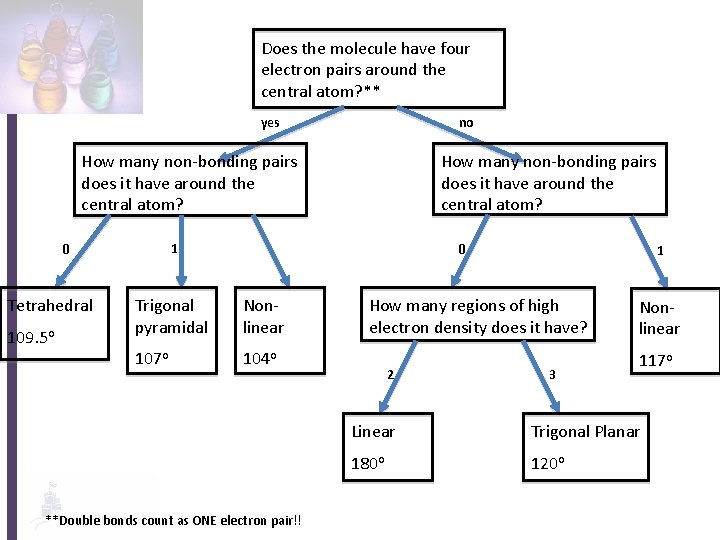
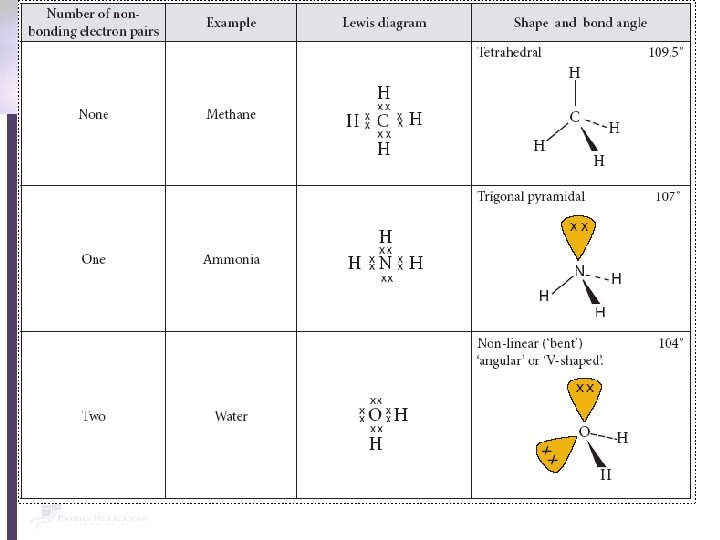
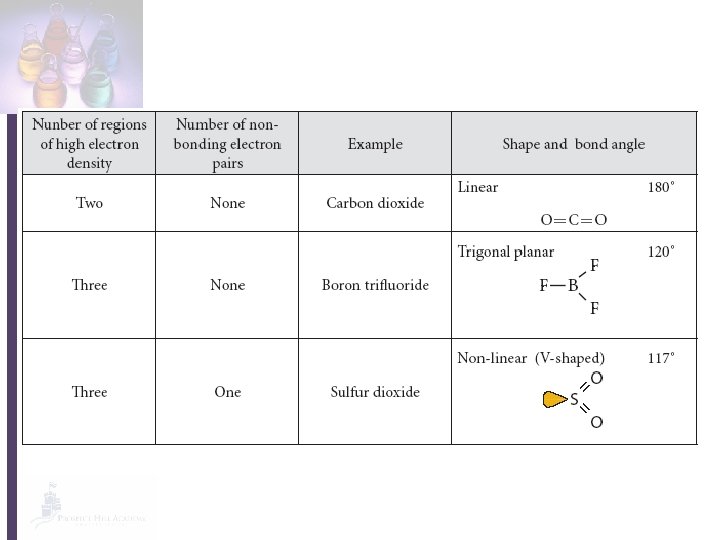
Does the molecule have four electron pairs around the central atom? ** yes no How many non-bonding pairs does it have around the central atom? 1 0 Tetrahedral 109. 5 o How many non-bonding pairs does it have around the central atom? 0 Trigonal pyramidal Nonlinear 107 o 104 o **Double bonds count as ONE electron pair!! 1 How many regions of high electron density does it have? 2 3 Nonlinear 117 o Linear Trigonal Planar 180 o 120 o

Tetrahedral Four electron pairs No non-bonding pairs 109. 5 o

Trigonal pyramidal Four electron pairs One non-bonding pair 107 o

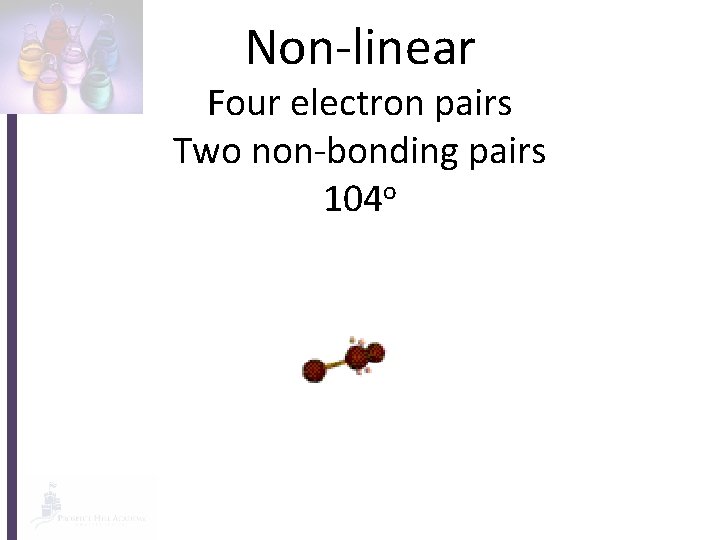
Non-linear Four electron pairs Two non-bonding pairs 104 o

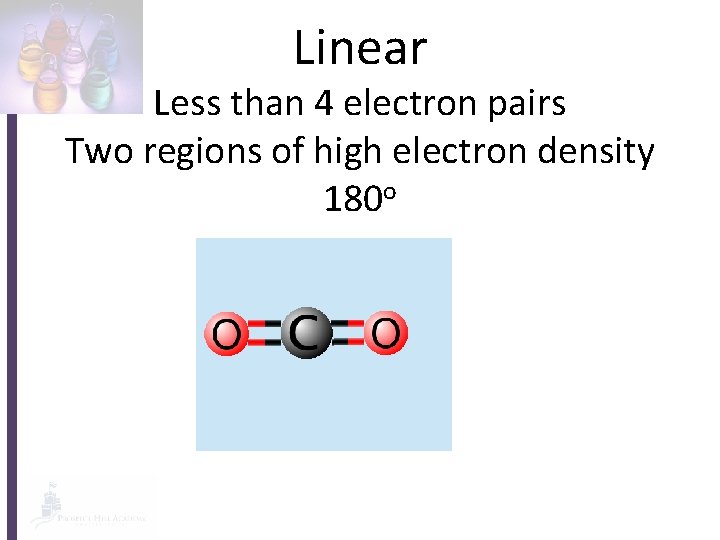
Linear Less than 4 electron pairs Two regions of high electron density 180 o

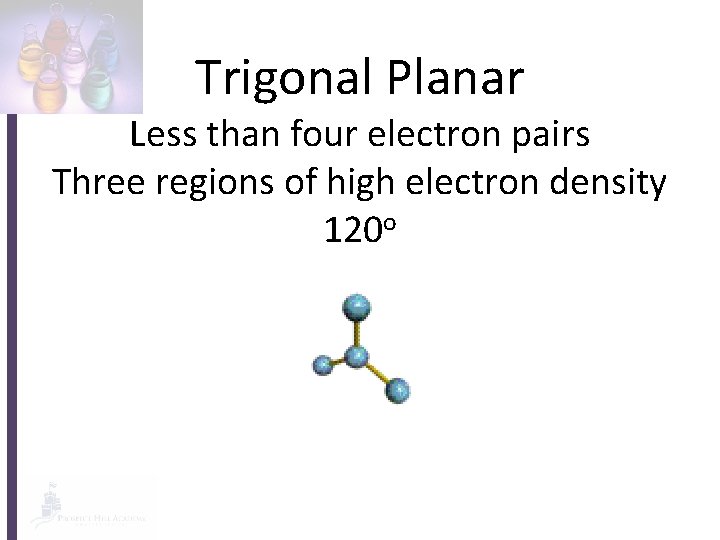
Trigonal Planar Less than four electron pairs Three regions of high electron density 120 o

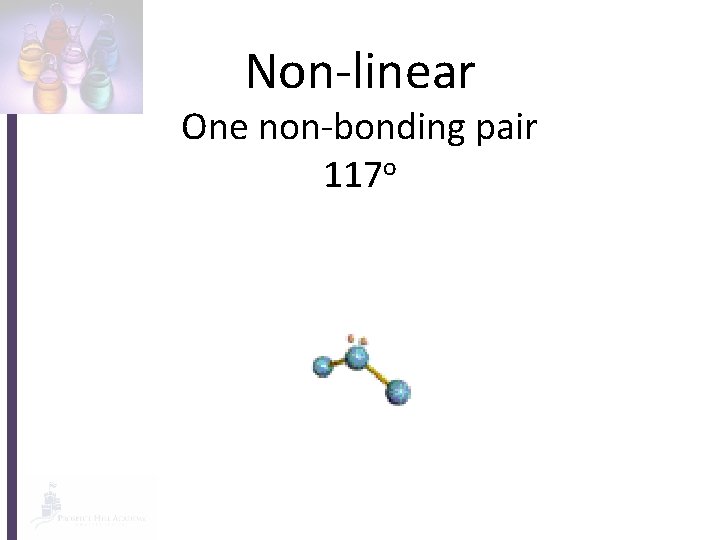
Non-linear One non-bonding pair 117 o



1. 2. 3. 4. 5. 6. CCl 4 CS 2 NF 3 BBr 3 H 2 S Se. O 2 1. Draw the Lewis Structure 2. Determine the shape using your flow chart, and write it on a piece of paper (1 per group). 3. Draw the shape and label the bond angle. 4. Place your gumdrop molecule under your work. 5. When you have finished all six, raise your hand!

Polarity

As a Group… Draw the Lewis Structures 1. 2. 3. 4. • CCl 4 HCCl 3 CO 2 H 2 O Identify and draw the shape of your molecules (use your flow chart)! • Then, build them from your molecule kit.

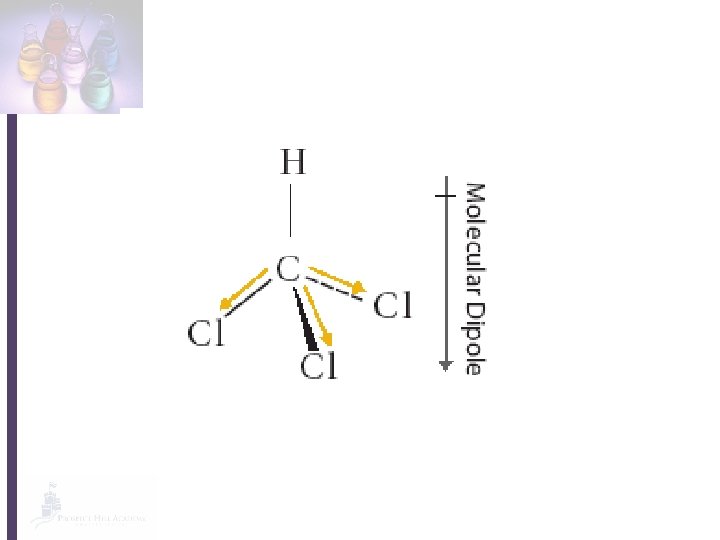
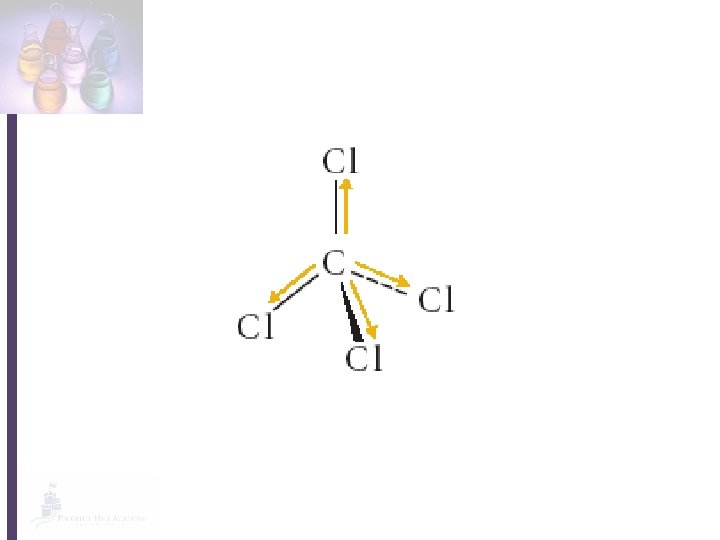
• In a covalent bond, the electrons are not always shared equally by the atoms. • The MORE electronegative atom will attract the electrons MORE. • More electronegative atom will have a slight negative charge. • Less electronegative atom will have a slight positive charge.

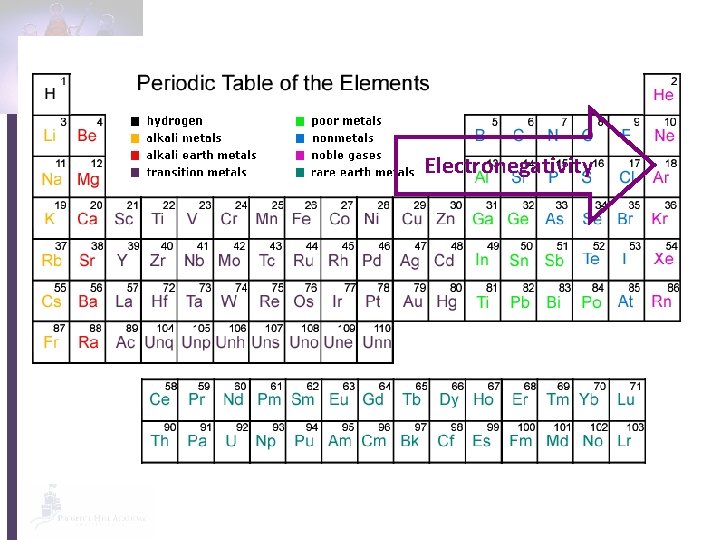
Electronegativity

Which will have the slight negative charge? 1. 2. 3. 4. 5. 6. N-O O-H C-F B-Cl S-I F-F

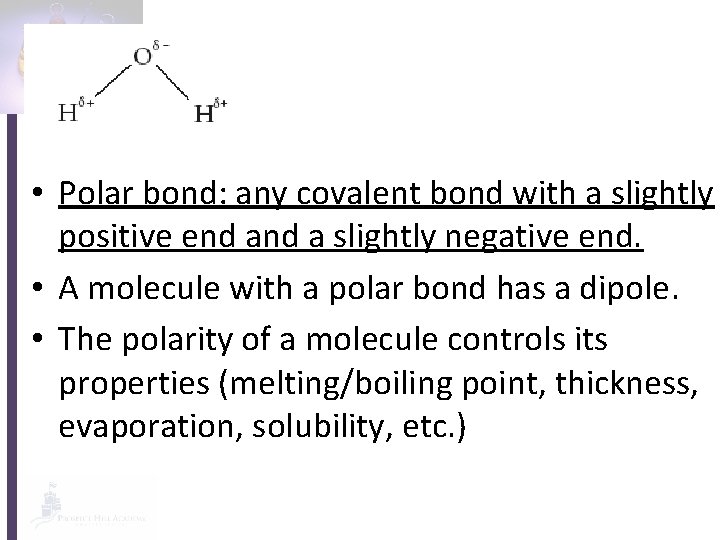
• Polar bond: any covalent bond with a slightly positive end a slightly negative end. • A molecule with a polar bond has a dipole. • The polarity of a molecule controls its properties (melting/boiling point, thickness, evaporation, solubility, etc. )

• Let’s find the polarity of your four molecules!

Practice • Draw Lewis Structures, shapes and label polarity for: • Si. F 4 • PCl 3 • H 2 S • NF 3 • Be. Cl 2 H 2 CO N 2 F 2

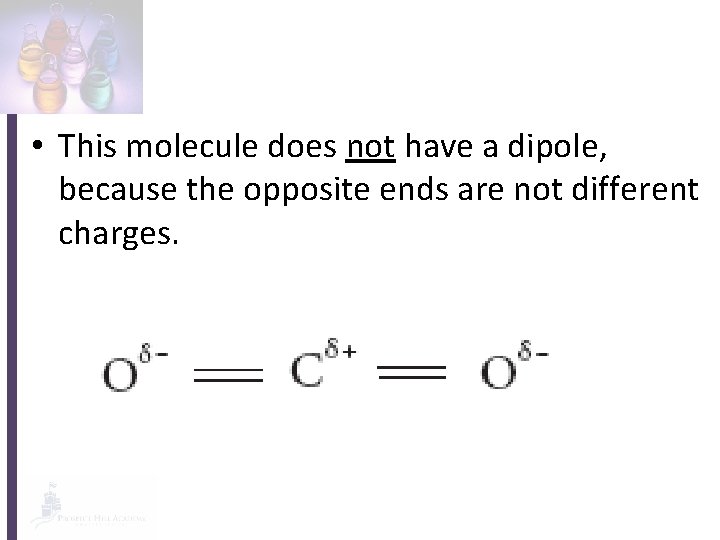
• This molecule does not have a dipole, because the opposite ends are not different charges.



• http: //www. youtube. com/watch? v=T 6 df. W 1 XW 3 FM