Salt Hydrolysis of Salts SALT Neutralization product of





- Slides: 13

Salt, Hydrolysis of Salts

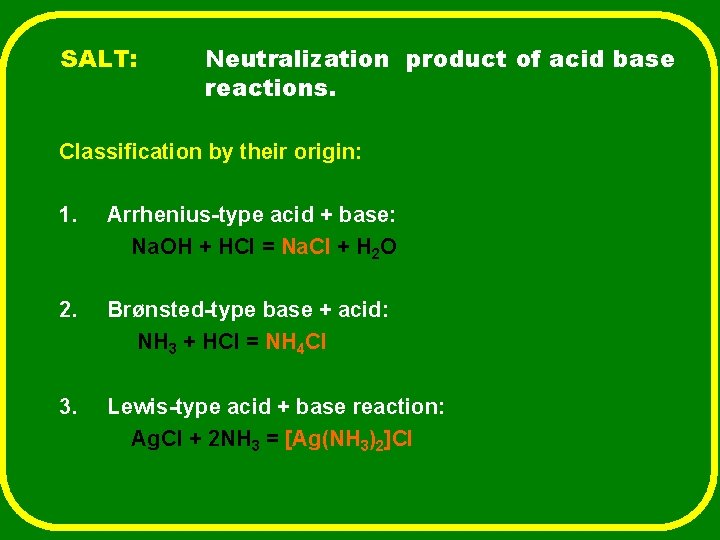
SALT: Neutralization product of acid base reactions. Classification by their origin: 1. Arrhenius-type acid + base: Na. OH + HCl = Na. Cl + H 2 O 2. Brønsted-type base + acid: NH 3 + HCl = NH 4 Cl 3. Lewis-type acid + base reaction: Ag. Cl + 2 NH 3 = [Ag(NH 3)2]Cl

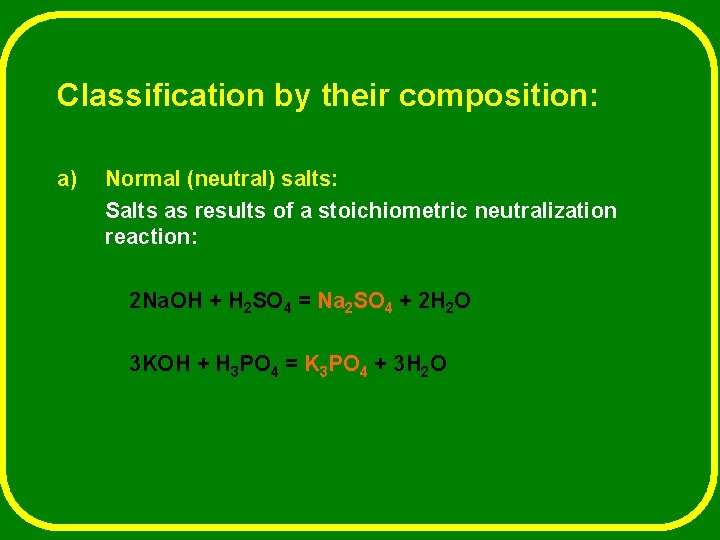
Classification by their composition: a) Normal (neutral) salts: Salts as results of a stoichiometric neutralization reaction: 2 Na. OH + H 2 SO 4 = Na 2 SO 4 + 2 H 2 O 3 KOH + H 3 PO 4 = K 3 PO 4 + 3 H 2 O

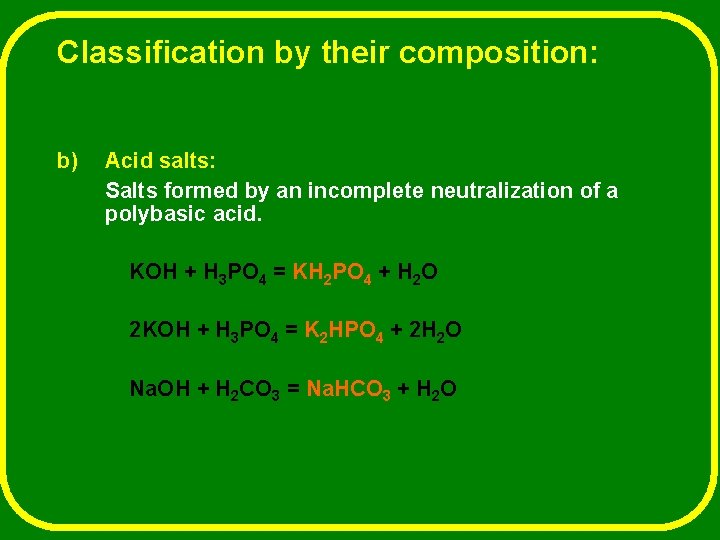
Classification by their composition: b) Acid salts: Salts formed by an incomplete neutralization of a polybasic acid. KOH + H 3 PO 4 = KH 2 PO 4 + H 2 O 2 KOH + H 3 PO 4 = K 2 HPO 4 + 2 H 2 O Na. OH + H 2 CO 3 = Na. HCO 3 + H 2 O

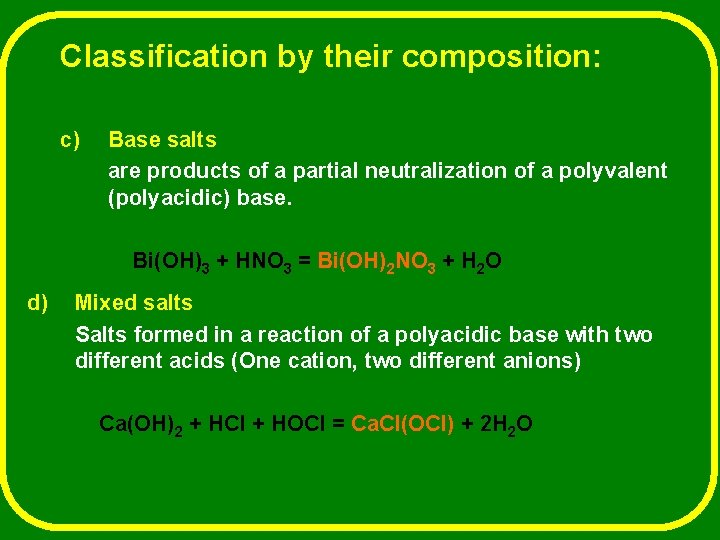
Classification by their composition: c) Base salts are products of a partial neutralization of a polyvalent (polyacidic) base. Bi(OH)3 + HNO 3 = Bi(OH)2 NO 3 + H 2 O d) Mixed salts Salts formed in a reaction of a polyacidic base with two different acids (One cation, two different anions) Ca(OH)2 + HCl + HOCl = Ca. Cl(OCl) + 2 H 2 O

e) Double salts: Composed of two different cations and one kind of anion K 2 SO 4 + Al 2(SO 4)3 = 2 KAl(SO 4)2 (alum) When dissolved, they dissociate into all of their ionic components: KAl(SO 4)2 = K+ + Al 3+ + 2 SO 42 or, e. g. : (NH 4)2 Fe(SO 4)2 (Mohr’s salt) When dissolved in water: (NH 4)2 Fe(SO 4)2 = 2 NH 4+ + Fe 2+ + 2 SO 42 -

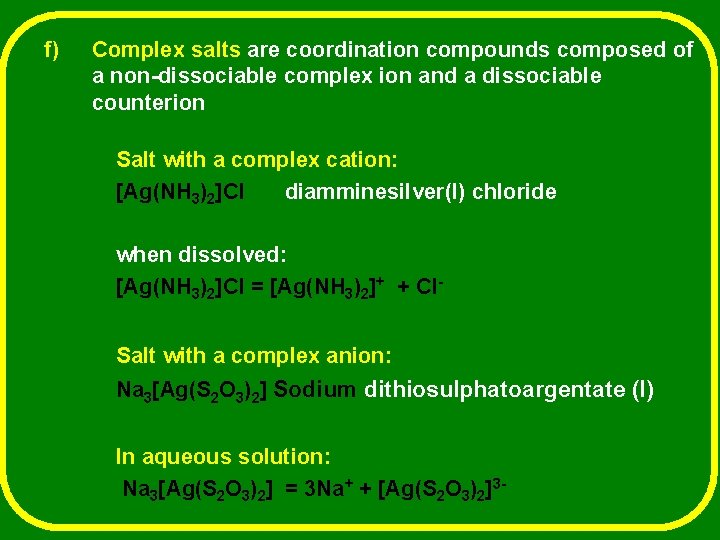
f) Complex salts are coordination compounds composed of a non-dissociable complex ion and a dissociable counterion Salt with a complex cation: [Ag(NH 3)2]Cl diamminesilver(l) chloride when dissolved: [Ag(NH 3)2]Cl = [Ag(NH 3)2]+ + Cl. Salt with a complex anion: Na 3[Ag(S 2 O 3)2] Sodium dithiosulphatoargentate (I) In aqueous solution: Na 3[Ag(S 2 O 3)2] = 3 Na+ + [Ag(S 2 O 3)2]3 -

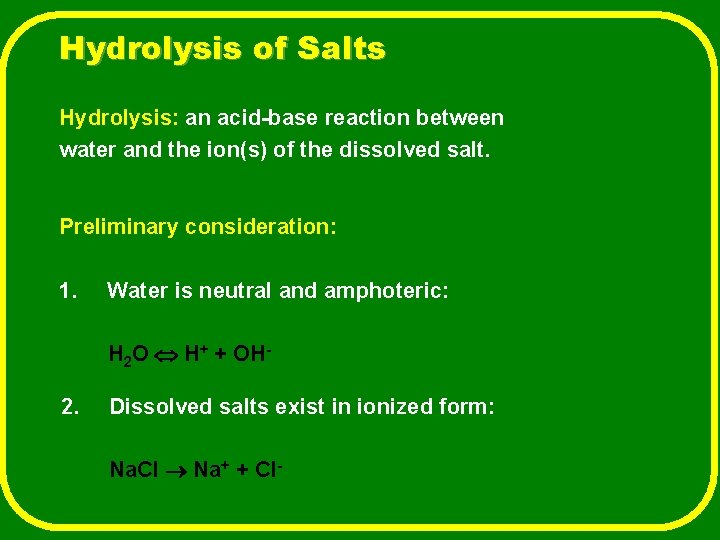
Hydrolysis of Salts Hydrolysis: an acid-base reaction between water and the ion(s) of the dissolved salt. Preliminary consideration: 1. Water is neutral and amphoteric: H 2 O H+ + OH- 2. Dissolved salts exist in ionized form: Na. Cl Na+ + Cl-

3. If any of the ions in solution has acid-base character, it will affect the self-ionization equilibrium of the solvent. 4. Cations of strong bases have no acid-base character while those of weak bases are acidic. K+ + H 2 O = N. R. NH 4+ + H 2 O NH 3 + H 3 O+ 5. Anions of strong acids have no acid-base character while those of weak asids are bases. SO 42 - + H 2 O = N. R. CN- + H 2 O HCN + OH-

Qualitative Aspects 1. No hydrolysis: Salts of strong acids and strong bases are neutral in solution. (Na. Cl, K 2 SO 4, Ca. Cl 2…. ) 2. Anion-hydrolysis: Salts of weak acids and strong bases are basic in solution. Dissolution: KCN K+ + CNHydrolysis: CN- + H 2 O HCN + OH-

Qualitative Aspects 3. Cation-hydrolysis: Salts of strong acids and weak bases are acidic in solution. Dissolution: NH 4 Cl NH 4+ + Cl. Hydrolysis: NH 4+ + H 2 O NH 3 + H 3 O+ 4. Cation-anion hydrolysis: Salts of weak acids and weak bases can be acidic, basic or neutral in solution, owing to the hydrolysis of both ions. The reaction depends on relative acid-base strengths. Dissolution: NH 4 CN NH 4+ + CNCation-Hydrolysis: NH 4+ + H 2 O NH 3 + H 3 O+ Anion-hydrolysis: CN- + H 2 O HCN + OH-

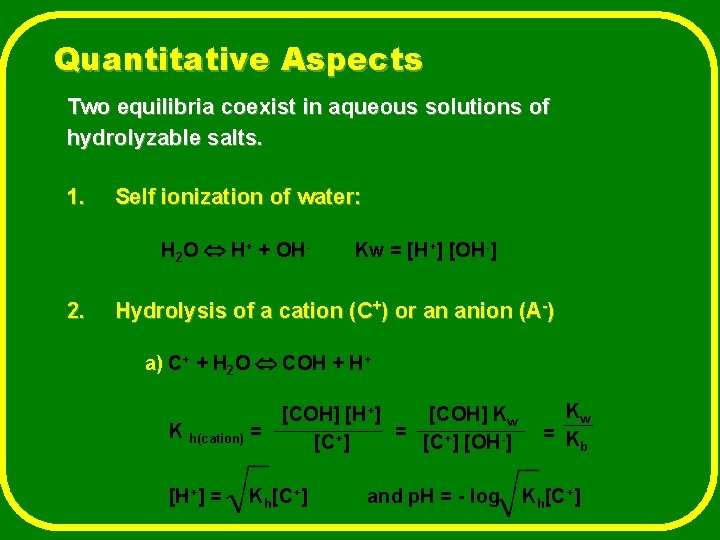
Quantitative Aspects Two equilibria coexist in aqueous solutions of hydrolyzable salts. 1. Self ionization of water: H 2 O H+ + OH- 2. Kw = [H+] [OH-] Hydrolysis of a cation (C+) or an anion (A-) a) C+ + H 2 O COH + H+ K h(cation) = [H+] = [COH] [H+] [COH] Kw = [C+] [OH-] Kh[C+] and p. H = - log Kw = K b Kh[C+]

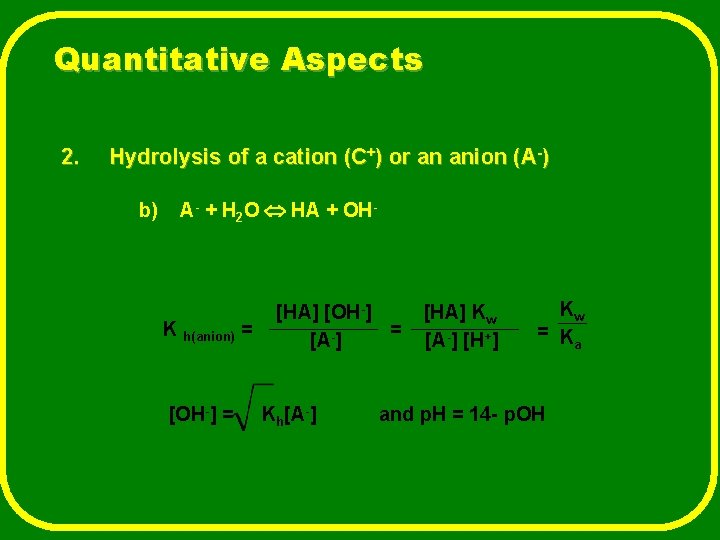
Quantitative Aspects 2. Hydrolysis of a cation (C+) or an anion (A-) b) A- + H 2 O HA + OH- K h(anion) = [OH-] = [HA] [OH-] = [A-] Kh[A-] [HA] Kw [A-] [H+] Kw = K a and p. H = 14 - p. OH
 Neutralization formula
Neutralization formula Toxin neutralization test
Toxin neutralization test Roman pasechnik
Roman pasechnik Writing neutralization reactions
Writing neutralization reactions Ionic equation for titration
Ionic equation for titration Titration formula
Titration formula Aso neutralization test
Aso neutralization test Neutralization titrations
Neutralization titrations Whats a neutralization reaction
Whats a neutralization reaction Hexagonal phospholipid neutralization
Hexagonal phospholipid neutralization Neutralization reaction
Neutralization reaction Combustion reaction
Combustion reaction Double tuned amplifier
Double tuned amplifier Neutralization reaction
Neutralization reaction