Routine use of pointofcare early infant diagnosis testing




- Slides: 16

Routine use of point-ofcare early infant diagnosis testing in Côte d’Ivoire: Early lessons learnt Patricia Fassinou Ekouévi IAS Satellite Session, 25 July 2017

Overview • HIV/AIDS in Côte d’Ivoire • Introducing point-of-care early infant diagnosis (POC EID) in Côte d’Ivoire • Operational progress • Results to date • Lessons learnt • Conclusions

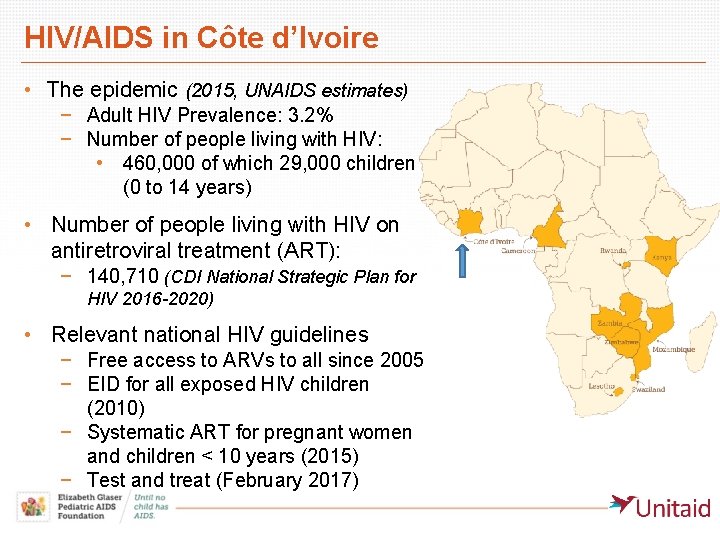
HIV/AIDS in Côte d’Ivoire • The epidemic (2015, UNAIDS estimates) − Adult HIV Prevalence: 3. 2% − Number of people living with HIV: • 460, 000 of which 29, 000 children (0 to 14 years) • Number of people living with HIV on antiretroviral treatment (ART): − 140, 710 (CDI National Strategic Plan for HIV 2016 -2020) • Relevant national HIV guidelines − Free access to ARVs to all since 2005 − EID for all exposed HIV children (2010) − Systematic ART for pregnant women and children < 10 years (2015) − Test and treat (February 2017)

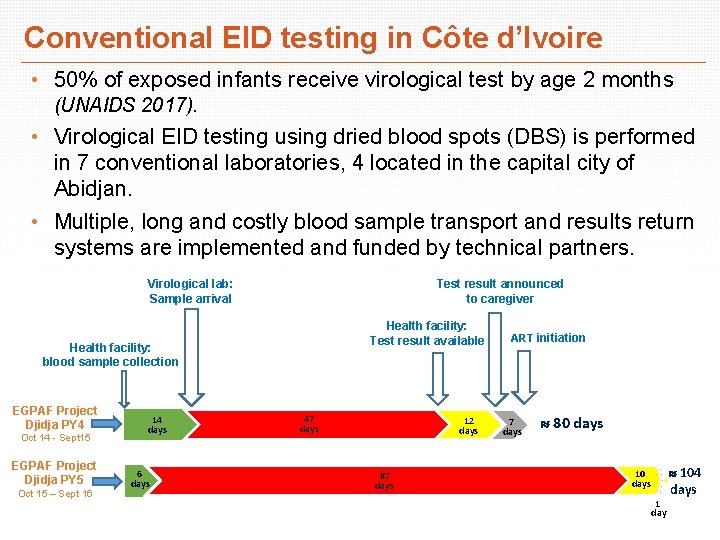
Conventional EID testing in Côte d’Ivoire • 50% of exposed infants receive virological test by age 2 months (UNAIDS 2017). • Virological EID testing using dried blood spots (DBS) is performed in 7 conventional laboratories, 4 located in the capital city of Abidjan. • Multiple, long and costly blood sample transport and results return systems are implemented and funded by technical partners. Virological lab: Sample arrival Test result announced to caregiver Health facility: Test result available Health facility: blood sample collection EGPAF Project Djidja PY 4 Oct 14 - Sept 15 EGPAF Project Djidja PY 5 Oct 15 – Sept 16 14 days 6 days 47 days 12 days 87 days ART initiation 7 days 80 days 1 day 104 days

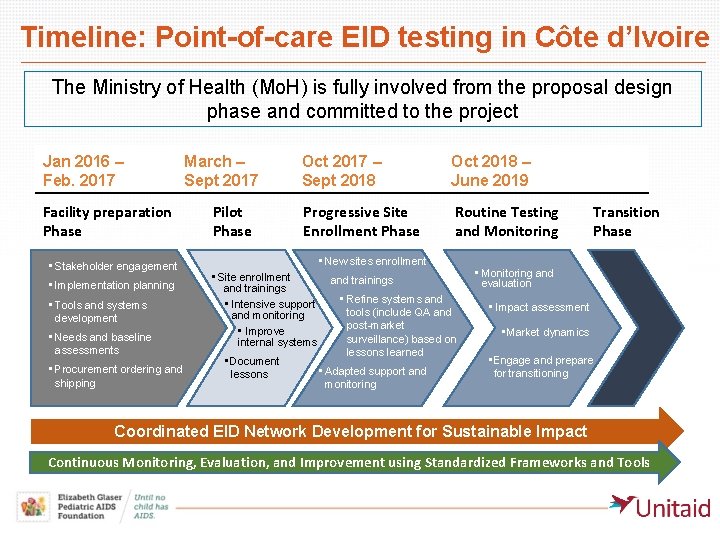
Timeline: Point-of-care EID testing in Côte d’Ivoire The Ministry of Health (Mo. H) is fully involved from the proposal design phase and committed to the project Jan 2016 – Feb. 2017 Facility preparation Phase • Stakeholder engagement • Implementation planning • Tools and systems development • Needs and baseline assessments • Procurement ordering and shipping March – Sept 2017 Pilot Phase Oct 2017 – Sept 2018 Oct 2018 – June 2019 Progressive Site Enrollment Phase Routine Testing and Monitoring • New sites enrollment • Site enrollment and trainings • Intensive support and monitoring • Improve internal systems • Document lessons and trainings • Refine systems and tools (include QA and post-market surveillance) based on lessons learned • Adapted support and monitoring Transition Phase • Monitoring and evaluation • Impact assessment • Market dynamics • Engage and prepare for transitioning Coordinated EID Network Development for Sustainable Impact Continuous Monitoring, Evaluation, and Improvement using Standardized Frameworks and Tools

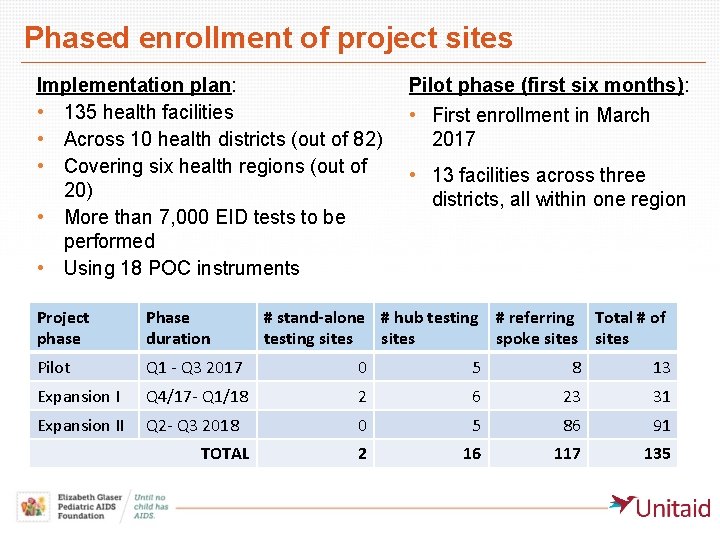
Phased enrollment of project sites Implementation plan: • 135 health facilities • Across 10 health districts (out of 82) • Covering six health regions (out of 20) • More than 7, 000 EID tests to be performed • Using 18 POC instruments Pilot phase (first six months): • First enrollment in March 2017 • 13 facilities across three districts, all within one region Project phase Phase duration Pilot Q 1 - Q 3 2017 0 5 8 13 Expansion I Q 4/17 - Q 1/18 2 6 23 31 Expansion II Q 2 - Q 3 2018 0 5 86 91 2 16 117 135 TOTAL # stand-alone # hub testing # referring Total # of testing sites spoke sites

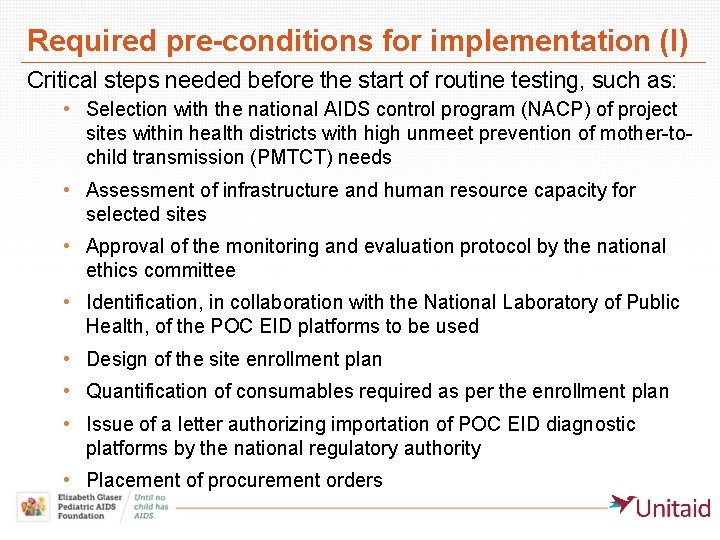
Required pre-conditions for implementation (I) Critical steps needed before the start of routine testing, such as: • Selection with the national AIDS control program (NACP) of project sites within health districts with high unmeet prevention of mother-tochild transmission (PMTCT) needs • Assessment of infrastructure and human resource capacity for selected sites • Approval of the monitoring and evaluation protocol by the national ethics committee • Identification, in collaboration with the National Laboratory of Public Health, of the POC EID platforms to be used • Design of the site enrollment plan • Quantification of consumables required as per the enrollment plan • Issue of a letter authorizing importation of POC EID diagnostic platforms by the national regulatory authority • Placement of procurement orders

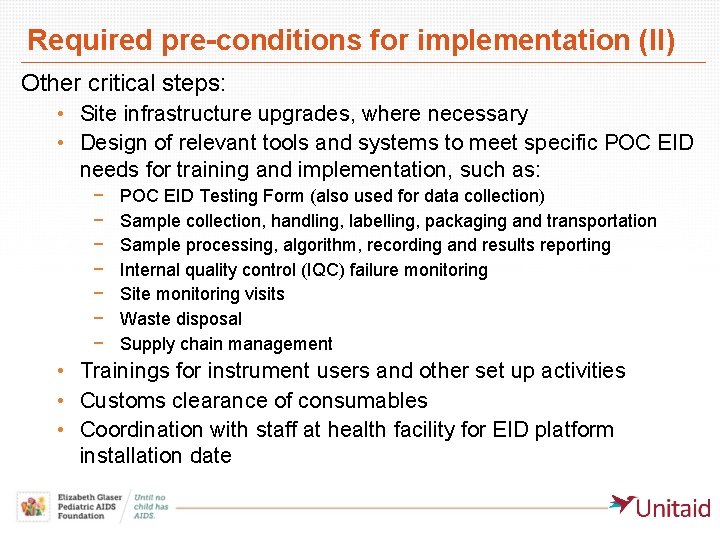
Required pre-conditions for implementation (II) Other critical steps: • Site infrastructure upgrades, where necessary • Design of relevant tools and systems to meet specific POC EID needs for training and implementation, such as: − − − − POC EID Testing Form (also used for data collection) Sample collection, handling, labelling, packaging and transportation Sample processing, algorithm, recording and results reporting Internal quality control (IQC) failure monitoring Site monitoring visits Waste disposal Supply chain management • Trainings for instrument users and other set up activities • Customs clearance of consumables • Coordination with staff at health facility for EID platform installation date

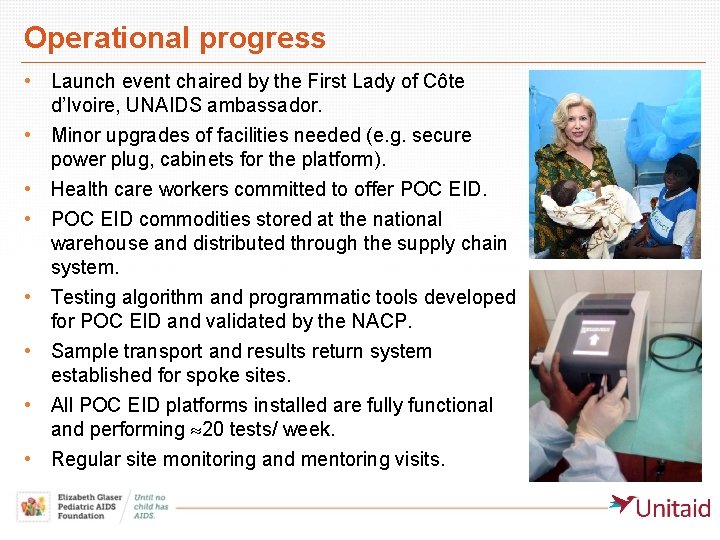
Operational progress • Launch event chaired by the First Lady of Côte d’Ivoire, UNAIDS ambassador. • Minor upgrades of facilities needed (e. g. secure power plug, cabinets for the platform). • Health care workers committed to offer POC EID. • POC EID commodities stored at the national warehouse and distributed through the supply chain system. • Testing algorithm and programmatic tools developed for POC EID and validated by the NACP. • Sample transport and results return system established for spoke sites. • All POC EID platforms installed are fully functional and performing 20 tests/ week. • Regular site monitoring and mentoring visits.

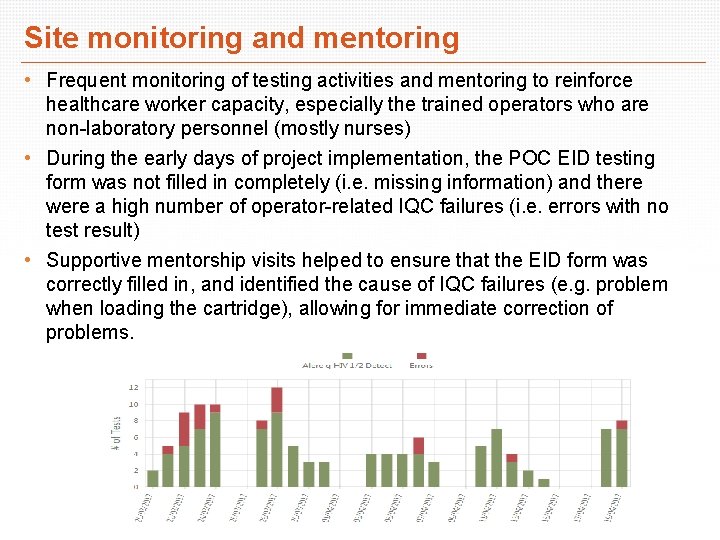
Site monitoring and mentoring • Frequent monitoring of testing activities and mentoring to reinforce healthcare worker capacity, especially the trained operators who are non-laboratory personnel (mostly nurses) • During the early days of project implementation, the POC EID testing form was not filled in completely (i. e. missing information) and there were a high number of operator-related IQC failures (i. e. errors with no test result) • Supportive mentorship visits helped to ensure that the EID form was correctly filled in, and identified the cause of IQC failures (e. g. problem when loading the cartridge), allowing for immediate correction of problems.

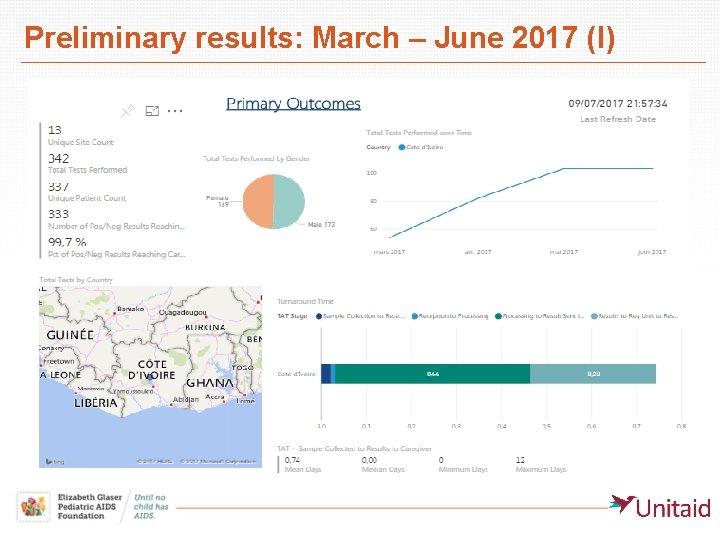
Preliminary results: March – June 2017 (I)

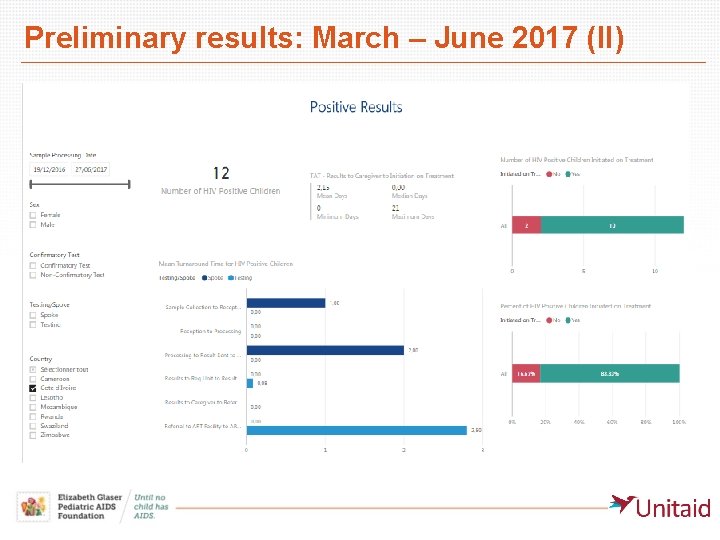
Preliminary results: March – June 2017 (II)

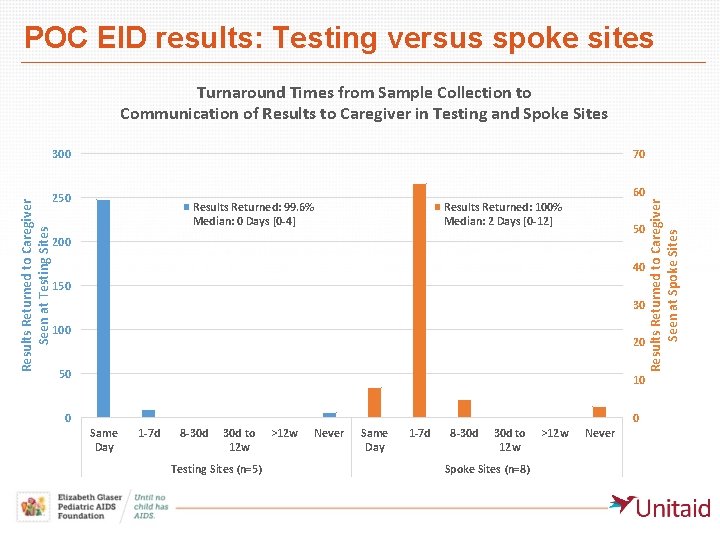
POC EID results: Testing versus spoke sites Turnaround Times from Sample Collection to Communication of Results to Caregiver in Testing and Spoke Sites 70 60 250 Results Returned: 99. 6% Median: 0 Days [0 -4] Results Returned: 100% Median: 2 Days [0 -12] 50 200 40 150 30 100 20 50 10 0 0 Same Day 1 -7 d 8 -30 d to 12 w Testing Sites (n=5) >12 w Never Same Day 1 -7 d 8 -30 d to 12 w Spoke Sites (n=8) >12 w Never Results Returned to Caregiver Seen at Spoke Sites Results Returned to Caregiver Seen at Testing Sites 300

Lessons Learnt • Endorsement by the Mo. H is crucial. • Site selection process must be coordinated with Mo. H. • Ownership of activities by health facility staff is key for introducing a new technology, for modifying processes, for task shifting etc. • Sound planning and pre-conditions are essential for smooth implementation of new technologies. • POC EID assays are easy to use, can be performed by non-laboratory staff given proper training and mentorship. • Frequent site monitoring required, with prompt investigation and corrective actions performed if necessary. • People living with HIV and HIV-positive women are receptive and enthusiastic about this new strategy that improves turnaround time of EID services. Blood sample collected at a spoke site. Test results automatically print on a portable printer.

Conclusions • POC EID preliminary results are encouraging and can serve as a model for strengthening access to prevention, care and treatment services for HIV/AIDS by continuously analyzing lessons learned from the project. • Step-wise site enrollment scheme is advised to allow all preconditions to be put in placed. • Bringing EID services closer to people is possible, and can be seen as a necessity to improve both health care and health outcomes. • EGPAF is engaged in eliminating HIV/AIDS because the science, the medicine, and the human resources are available; we just need to bring them together.

Thank you! Merci!
 Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Types of nursing diagnoses
Types of nursing diagnoses Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Early cpr and early defibrillation can: *
Early cpr and early defibrillation can: * What is domain
What is domain Logic based testing in software testing
Logic based testing in software testing Du path testing
Du path testing Positive and negative testing
Positive and negative testing Cs3250
Cs3250 Globalization testing in software testing
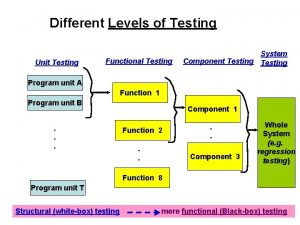
Globalization testing in software testing Functional testing vs unit testing
Functional testing vs unit testing Cause effect graphing technique
Cause effect graphing technique Control structure testing in software testing
Control structure testing in software testing Decision table testing in software testing
Decision table testing in software testing What is decision table testing
What is decision table testing