Comparing Conventional to PointofCare POC Early Infant Diagnosis





- Slides: 18

Comparing Conventional to Point-of-Care (POC) Early Infant Diagnosis (EID): Pre and post intervention data from a multicountry evaluation. Flavia Bianchi, Valery Nzima, Addmore Chadambuka, Anafi Mataka, Gcinile Nyoni, Gilles Ndayisaba, Patricia Fassinou, Rhoderick Machekano, Emma Sacks, Rebecca Bailey, Rebecca Alban, Jean-François Lemaire, Jennifer Cohn IAS Satellite Session Paris, France July 25, 2017

The authors have no conflicts to declare.

Overview Background and rationale for POC EID project Baseline data (6 countries) Interim data from POC use (6 countries) Comparing conventional and POC EID on key service delivery indicators • Conclusions • •

Background • Survival of HIV-infected infants depends on a robust early infant diagnosis (EID) system • Turnaround time for conventional EID is typically between 30 -90 days • HIV infected, untreated infants have rapid disease progression and high mortality • Half of HIV-infected infants will die by age 2 if untreated • Integration of point-of-care (POC) EID testing into a country’s existing laboratory network could improve EID system functioning.

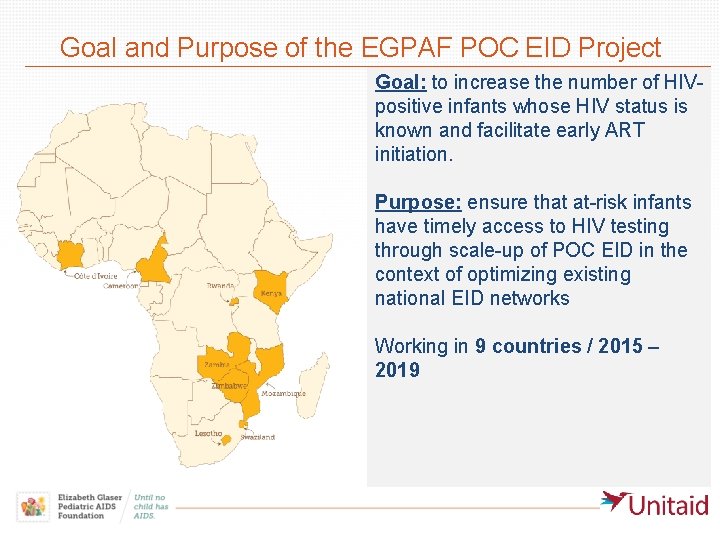
Goal and Purpose of the EGPAF POC EID Project Goal: to increase the number of HIVpositive infants whose HIV status is known and facilitate early ART initiation. Purpose: ensure that at-risk infants have timely access to HIV testing through scale-up of POC EID in the context of optimizing existing national EID networks Working in 9 countries / 2015 – 2019

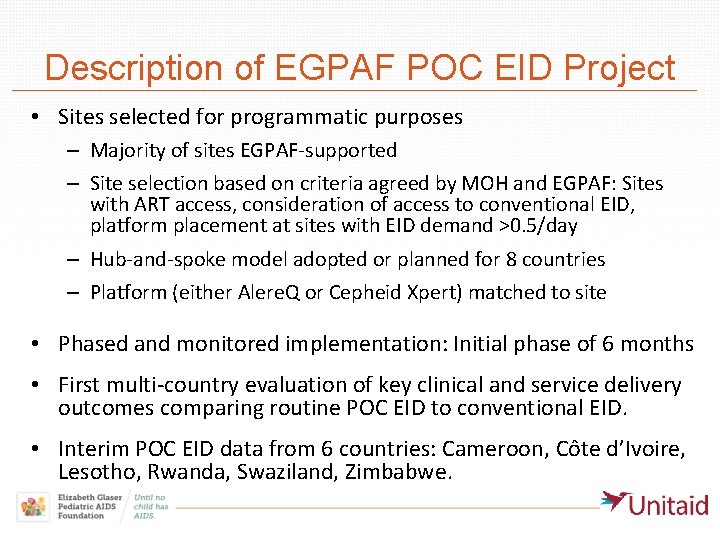
Description of EGPAF POC EID Project • Sites selected for programmatic purposes – Majority of sites EGPAF-supported – Site selection based on criteria agreed by MOH and EGPAF: Sites with ART access, consideration of access to conventional EID, platform placement at sites with EID demand >0. 5/day – Hub-and-spoke model adopted or planned for 8 countries – Platform (either Alere. Q or Cepheid Xpert) matched to site • Phased and monitored implementation: Initial phase of 6 months • First multi-country evaluation of key clinical and service delivery outcomes comparing routine POC EID to conventional EID. • Interim POC EID data from 6 countries: Cameroon, Côte d’Ivoire, Lesotho, Rwanda, Swaziland, Zimbabwe.

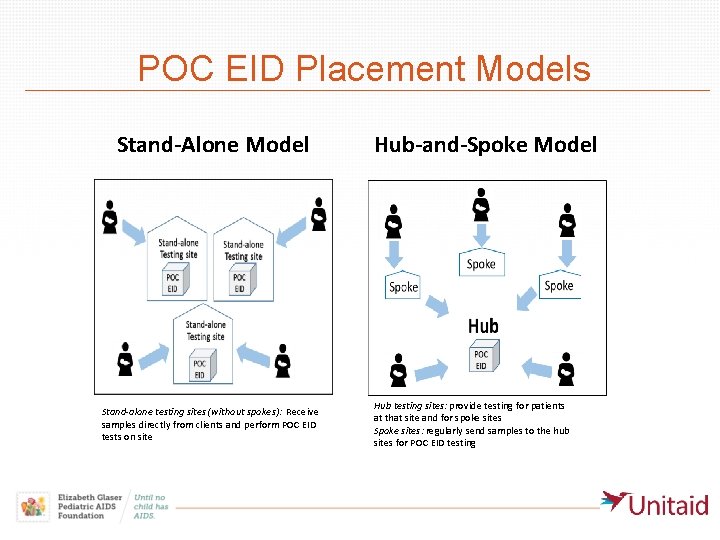
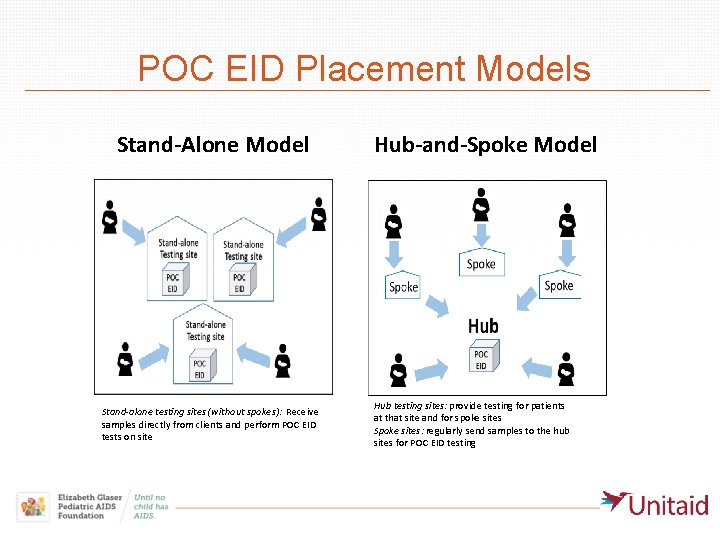
POC EID Placement Models Stand-Alone Model Stand-alone testing sites (without spokes): Receive samples directly from clients and perform POC EID tests on site Hub-and-Spoke Model Hub testing sites: provide testing for patients at that site and for spoke sites Spoke sites: regularly send samples to the hub sites for POC EID testing

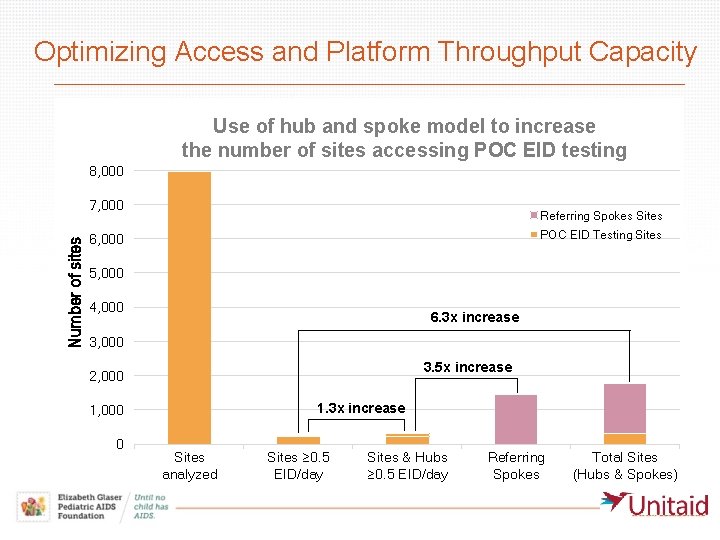
Optimizing Access and Platform Throughput Capacity Use of hub and spoke model to increase the number of sites accessing POC EID testing 8, 000 Number of sites 7, 000 Referring Spokes Sites POC EID Testing Sites 6, 000 5, 000 4, 000 6. 3 x increase 3, 000 3. 5 x increase 2, 000 1. 3 x increase 1, 000 0 Sites analyzed Sites ≥ 0. 5 EID/day Sites & Hubs ≥ 0. 5 EID/day Referring Spokes Total Sites (Hubs & Spokes)

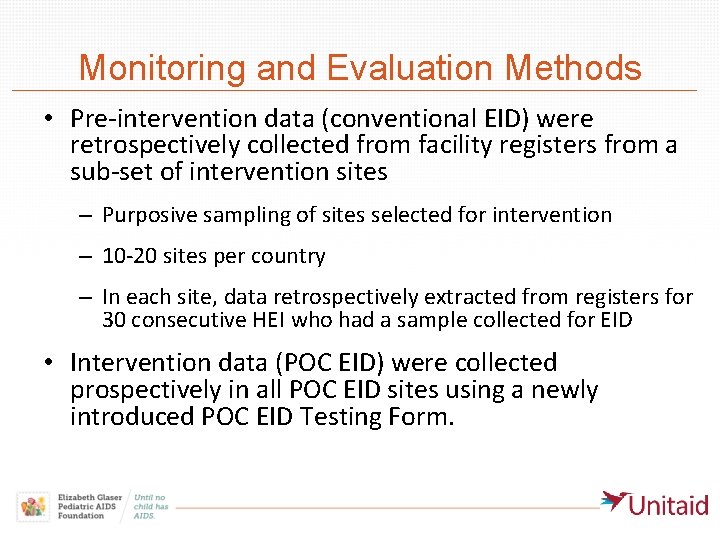
Monitoring and Evaluation Methods • Pre-intervention data (conventional EID) were retrospectively collected from facility registers from a sub-set of intervention sites – Purposive sampling of sites selected for intervention – 10 -20 sites per country – In each site, data retrospectively extracted from registers for 30 consecutive HEI who had a sample collected for EID • Intervention data (POC EID) were collected prospectively in all POC EID sites using a newly introduced POC EID Testing Form.

Conventional Laboratory EID Results for Primary Evaluation Outcomes Country (number of conventional tests) % Results Median number of received days [range] from by Infant blood collection to Caregiver return of results to caregiver * # of HIVMedian number of days infected [range] between receipt of infants results by caregiver and initiated on initiation on treatment of ART HIV-infected infant Cameroon (n= 240) 49% 35 days [9 -381] 10/14 0 days [0 -22] CDI (n= 315) 64% 81 days [2 -234] 4/5 0 days [0 -32] Lesotho (n= 269) 82% 63 days [13 -410] 7/8 0 days [0 -0] Rwanda (n=600) 93% 34 days [3 -366] 8/9 27 days [0 -75] Swaziland (n= 180) 61% 31 days [9 -104] 0/2 N/A Zimbabwe (n = 606) 83% 61 days [16 -438] 21/31 0 days [0 -6] 77. 4% 53 days [2 -438] 72. 4% (50/69) 0 days [0 -75] Total (n= 2210 at 73 sites) *Tests for which no date for caregiver result return was recorded were censored and not included in calculation of turn-around-time.

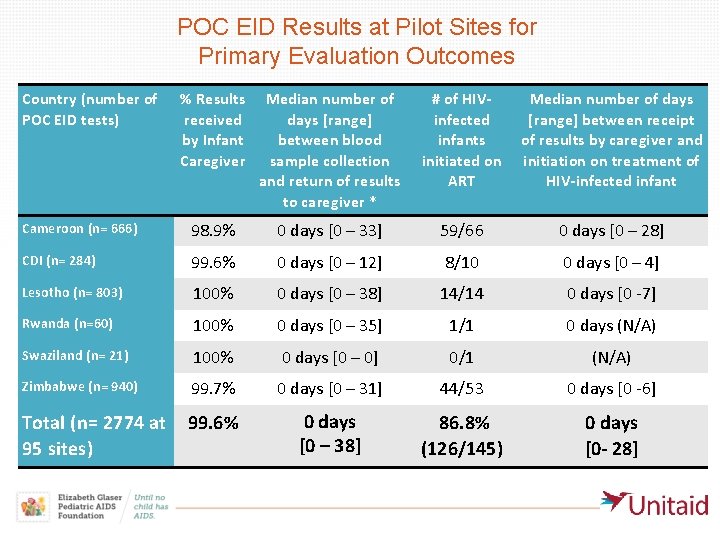
POC EID Results at Pilot Sites for Primary Evaluation Outcomes Country (number of % Results POC EID tests) received by Infant Caregiver Median number of days [range] between blood sample collection and return of results to caregiver * # of HIVMedian number of days infected [range] between receipt infants of results by caregiver and initiated on initiation on treatment of ART HIV-infected infant Cameroon (n= 666) 98. 9% 0 days [0 – 33] 59/66 0 days [0 – 28] CDI (n= 284) 99. 6% 0 days [0 – 12] 8/10 0 days [0 – 4] Lesotho (n= 803) 100% 0 days [0 – 38] 14/14 0 days [0 -7] Rwanda (n=60) 100% 0 days [0 – 35] 1/1 0 days (N/A) Swaziland (n= 21) 100% 0 days [0 – 0] 0/1 (N/A) Zimbabwe (n= 940) 99. 7% 0 days [0 – 31] 44/53 0 days [0 -6] 0 days Total (n= 2774 at 99. 6% 86. 8% 0 days [0 – 38] *Tests for which no date for caregiver result return was recorded were censored and not included in calculation of turn-around-time. 95 sites) (126/145) [0 - 28]

POC EID Placement Models Stand-Alone Model Stand-alone testing sites (without spokes): Receive samples directly from clients and perform POC EID tests on site Hub-and-Spoke Model Hub testing sites: provide testing for patients at that site and for spoke sites Spoke sites: regularly send samples to the hub sites for POC EID testing

Comparing Testing and Spoke Sites Indicator Testing Sites (n = 28 sites) Spoke Sites (n = 67 sites) % of results returned to caregiver 99. 5% 99. 9% Median turnaround time from blood sampling to caregiver receipt of results 0 days (range: 0 -33 days) 2 days (range: 0 -38 days) Median turnaround time from receipt of results to initiation on treatment 0 days (range: 0 -28 days) 0 days (range: 0 -7 days) % HIV-Infected children initiated on treatment 88. 5% 80. 6%

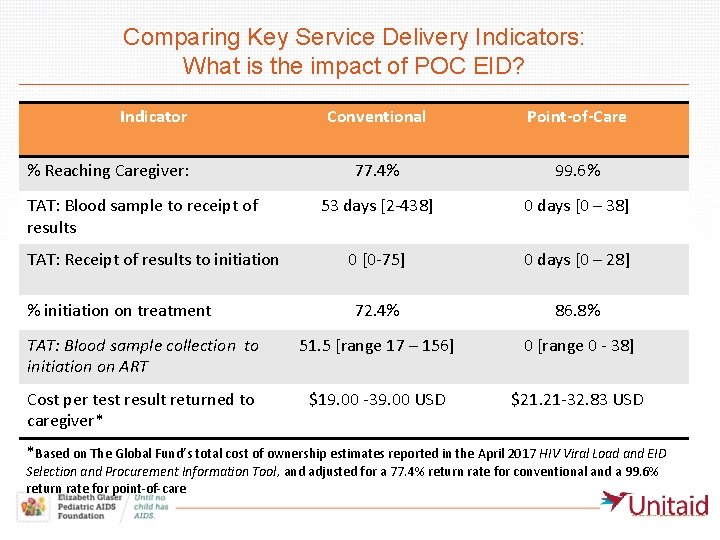
Comparing Key Service Delivery Indicators: What is the impact of POC EID? Indicator Conventional Point-of-Care 77. 4% 99. 6% 53 days [2 -438] 0 days [0 – 38] 0 [0 -75] 0 days [0 – 28] 72. 4% 86. 8% TAT: Blood sample collection to initiation on ART 51. 5 [range 17 – 156] 0 [range 0 - 38] Cost per test result returned to caregiver* $19. 00 -39. 00 USD $21. 21 -32. 83 USD % Reaching Caregiver: TAT: Blood sample to receipt of results TAT: Receipt of results to initiation % initiation on treatment *Based on The Global Fund’s total cost of ownership estimates reported in the April 2017 HIV Viral Load and EID Selection and Procurement Information Tool, and adjusted for a 77. 4% return rate for conventional and a 99. 6% return rate for point-of-care

Cost Per Test Returned • The price of diagnostic technologies for EID – both conventional and POC – should be a key consideration • Currently, the individual test price is higher for POC EID than for conventional EID • However, it is important to not only consider the cost of the test itself but also comprehensive operating costs of delivering EID testing services, as well as the rate of results return to caregivers • Cost per test result received may be considered a closer measure of the true value of a diagnostic • Cost per test result received are estimated to be approximately $19. 00 -39. 00 USD and $21. 21 -32. 83 USD for conventional and POC, respectively.

Limitations • We rely on routinely collected data; baseline data was preexisting data…data quality remains an issue. But very invested in data quality assurance…watch this space. • The majority of sites are EGPAF sites, potential for bias. • Sampling of baseline sites was purposive…considered size of facility, volume, urban/rural. • Impact evaluation is forthcoming to help address some of these barriers.

Conclusions • POC EID shows improvement over conventional centralized testing on service delivery outcomes such as: § A greater proportion of infants obtained their results § Infants receive their results on the same day, rather than waiting 53 days § A higher proportion of HIV+ infants get started on ART § Same day ART initiation still observed (no delay) • Sample transportation over small distances (from spoke to hub sites) allows for similar performance than when patient is seen at testing site, and may be considered to increase access to EID • Cost per test result received by caregiver is similar between POC and conventional testing • HIV programs should consider use of POC EID to improve EID outcomes.

Thank you
 Nursing process objectives
Nursing process objectives What are the nursing process
What are the nursing process Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Second phase of nursing process
Second phase of nursing process Onet database
Onet database Poc componentes
Poc componentes Poeif
Poeif Poc analyzátorů
Poc analyzátorů Pentamin
Pentamin Poc informatics systems
Poc informatics systems Ohayo poc
Ohayo poc Vdp ibm
Vdp ibm Poc project management
Poc project management Point of confusion avid examples
Point of confusion avid examples Poc
Poc Poc datenmanagementsystem
Poc datenmanagementsystem Analyzátorů krevních plynů poc
Analyzátorů krevních plynů poc Proof of concept project plan
Proof of concept project plan