Research at Eskenazi Health An EPIC Overview of




























- Slides: 39

Research at Eskenazi Health: An EPIC Overview of Research Policies and Procedures Version Date: 11. 09. 16

Outline • • Meet the Team Eskenazi Health Research Policy EH Grants EH Research Application EH Researcher Credentialing EH Research Billing How to Close an EH Study EPIC EMR

Meet the Team • Legal • • • Research Billing • • • Nina Brahm, Grants Director Shawn Wellman, Grants Administrator Medical Records - EPIC – Research • • • Tammy Shoultz, Medical Staff Affairs Director Suzanne Maxwell, Medical Staff Affairs Coordinator Grants Department • • • Gail Jenkins, Billing Manager Kayla Bullock, PFS Receivable Analyst Medical Staff Affairs • • • Phyllis Garrison, JD, Privacy Director Andrea Heid, Legal Administration & Contracts Manager John Putz, Clinical Research Manager Justin Morea, DO, MS, Associate CMIO Kris Elkins, Senior Clinical Epic Analyst Jen Hillstrom, Research Principal Trainer Researcher Consultants • • • David Haas, MD, Physician Nikki Mehdiyoun, Clinical Research Manager Megan Gaunnac, Operations Specialist

We are Here for You! • Over the years, we received feedback regarding the process • Research Compliance Committee was formed in early 2014 to start tackling researcher concerns. To outline a few: • • Lack of clear policy and procedures Research billing and invoicing difficulties Linkages to the correct EH staff members - troubleshooting Researcher access to EMR • We hope the updated process will satisfy the needs of research teams, but it is a work in progress. We are a volunteer group. Be patient!

Materials from Today’s Presentation • All information/forms from today’s presentation is available on the IU Clinical Trials Office (CTO) website: https: //indianaclinicaltrialsoffice. iu. edu/investigators-studycoordinators/eskenazi-health/

Eskenazi Health Research Policy • Institutional Research Protections, 950 -87 • Available on the IU Clinical Trials Office (CTO) website: https: //indianaclinicaltrialsoffice. iu. edu/investigators-study-coordinators/eskenazi-health/ • Policy applies to ALL RESEARCH conducted at Eskenazi Health, with Eskenazi Health patients, or utilizing Eskenazi Health medical records • Highlights – PRIOR to commencing study procedures: • All research conducted at Eskenazi must receive EH approval • Must have IU IRB approval • If any Eskenazi Health campus (EH, H&H, Midtown, etc) is listed as a performance site, expect that at your next IRB continuing review or new study submission you will be prompted to obtain EH research approval • All staff conducting research/reviewing medical records, must be approved by Medical Staff Affairs • The policy is to aid research, not obstruct it. EH will approve ongoing research and grandfather existing research IF all policies and procedures are completed!

EH Research Approval Process • One stop shop for all required procedures to be in compliance with the policies • A new application and approval process is implemented! – – https: //redcap. uits. iu. edu/surveys/? s=APYTFHTXML “Request to Conduct Research at Eskenazi Health” REDCap survey Web access – Anyone can submit application; however it is suggested that the primary study coordinator submits • Must submit one application for EACH research study

Oh No! Not another approval! • Why? – The previous procedure lacked sufficient information to determine various research conducted at EH, with EH patients, or with EH medical records – The new application will aid in speedier research services • Have no fear…there is not a complicated review process! • EH mandates IU IRB approval (or a waiver, if applicable)

EH GRANTS

EH Grants • Will EH staff effort be used to carry out any aspect of the clinical research? – If YES, you MUST make the request through the Grants Department • Instructions for Requesting Use of EH Staff • Email request to EHGrants@eskenazihealth. edu – Letter of Support (if available) previously provided by Eskenazi Health administration. Include in body of email the name, email and phone for Principal Investigator and Research Coordinator – Completed Request to Use Eskenazi Health Staff form • All requests for Eskenazi Health staff effort in any aspect of clinical research, and/or grant-funded projects conducted by non-Eskenazi Health entities, MUST be approved by Eskenazi Health Administration prior to effort being performed

EH RESEARCH APPLICATION

Research Application: Nuts & Bolts • • • When does the application need to be completed? – The application needs to be completed for all human subjects research (including that which is not intended for public dissemination) conducted on Eskenazi Health property, interventions with Eskenazi Health patients, or accessing Eskenazi Health patient PHI – One for each study Complete the application at: https: //redcap. uits. iu. edu/surveys/? s=APYTFHTXML You do not have to have a REDCap account in order to complete the application Preference is for the primary study coordinator to complete the application You are able to save & return if you need to – Do not just close the browser, click the “save & return” button on the bottom of the page – This will provide you with a code for returning to the survey (write this down because you will need it) • • Once you submit your application, the screen will state “Eskenazi Health: Here for You Thank You!” If you have any issues with the application, email research 1@eskenazihealth. edu

Research Application: Levels of Research • • If you meet criteria for more than one study level, choose the lowest numbered level, which is the most inclusive Levels: – Level 1. Conducting full study procedures on Eskenazi Health campuses (including providing an intervention OR completing procedures on Eskenazi Health campuses) – Level 2. Biospeciman research ONLY (collection of Eskenazi Health patient biospecimans) – Level 3. Observational/naturalistic study – Level 4. Medical Record review of Eskenazi Health records ONLY for retrospective or prospective reviews (no interaction between subject and researcher on Eskenazi Health property [including epidemiological studies, outcome studies, etc. ] – Level 5. Recruitment ONLY (no study procedures will be completed on Eskenazi Health property, may include medical record reviews -- Informed consent only is considered in this level) – Level 6. Student/Resident/Intern project at Eskenazi Health (no publishing, no external presentations, has an Eskenazi Health Medical Staff mentor and is currently engaged in an Eskenazi Health approved educational program) – Level 7. Anonymous or de-identified survey ONLY (no PHI will be collected)

Research Application: Mandatory Info Prior to starting the application, have the following handy: – – – – PI Contact Information Date of Initial IU IRB approval Most recent IU IRB Approval Letter (upload required) Current IU IRB approved Protocol (upload required) Most recent IU IRB Protocol Summary w/ Eskenazi Health listed as a study site (upload required) NCT# Basic study information Plan for Eskenazi Health specific information including: • Anticipated number of Eskenazi Health patients (or medical records) involved in research • Eskenazi Health locations where research will be conducted (i. e. . Primary Care, Emergency Department, etc) • Recruitment of Eskenazi Health patients/HIPAA – – – Basic investigational medications/devices information All IU IRB approved “Drug & Biological Products” Forms (if applicable) All IU IRB approved “Medical Device” Forms (if applicable) Research Billing Grid/Separation of Allowable Costs at Eskenazi Health Completed “Research Projects Conducted at Eskenazi Health” spreadsheet (upload required) All applicable forms for new access/researcher requests (Research Credentialing Applicant Information Form , CIRA, Flu, & TB) (upload required)

Research Application: Timeline • Complete Research Application – IU IRB approved documents are required • EH approval must be received prior to commencing study procedures conducted on EH property, interventions with EH patients, or accessing EH patient PHI for research purposes

Approvals: Ongoing & New Studies • Submit a Research Application for ALL RESEARCH, regardless whether ongoing or a new research study • Submit one application for each research study • Starting today, this new process will replace all existing processes • Ensure your research staff are trained on the updated policies and procedures and are aware of the changes • Note: All research studies will need to be registered in EPIC that are medically relevant or have research billing.

EH RESEARCH CREDENTIALING

EH Research Credentialing • All research personnel conducting research at EH are required to obtain researcher credentials (in addition to medical staff credentialing) • Personnel are only required to obtain research credentialing one time (vs. for each research study) • To maintain researcher credentialing, all personnel must complete the annual re-credentialing process (submit annual TST and flu shot) • Two types of researchers • Medical Credentialing plus Researcher • Researcher only

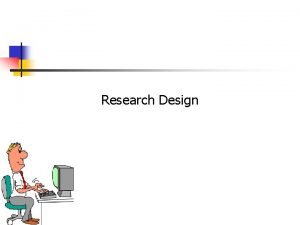
EH Credentialing (cont. ) • Ongoing access • Complete the "Research Projects Conducted at Eskenazi Health" spreadsheet • New access • Steps for credentialing: – Submit the following for EACH person requesting research credentialing: • Eskenazi Health Confidentiality and Information Resource Agreement • Research Credentialing Applicant Information Form • Copy of most recent TST (must have been completed within the past year) • Copy of most recent Flu Shot (if you have not received the flu shot within the past 12 months, this requirement will be waived until the subsequent fall when flu shots are offered) – Complete the "Research Projects Conducted at Eskenazi Health" spreadsheet – Submit all of the above to the Medical Staff Affairs office: Suzanne. maxwell@eskenazihealth. edu

EH Credentialing (cont. ) Even though multiple studies are listed on the Master List, a separate application must be completed and approved for each study prior to commencement Anytime someone is added or removed from a study, update the spreadsheet and submit it to the Medical Staff Affairs office: Suzanne. maxwell@eskenazihealth. edu

EH RESEARCH BILLING & EPIC EMR

What Role Does the Epic EMR Play in the Research Process? • Improved clinical care for patients involved in research trials – EMR notification of research status – Notification of ADT events and missed appointments • Streamlined research billing procedures • Advanced reporting capabilities • Improved clinical research workflow efficiency

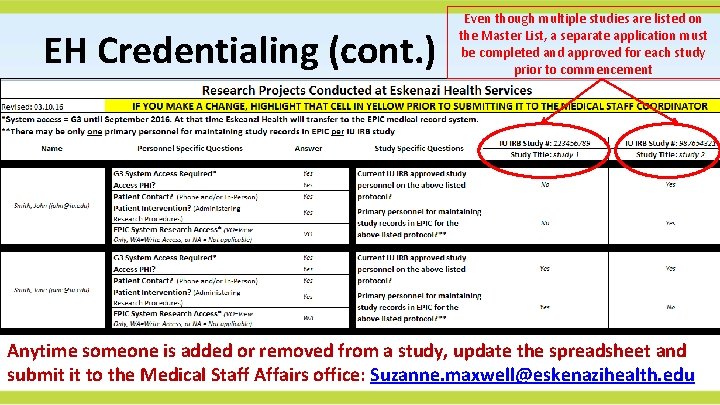
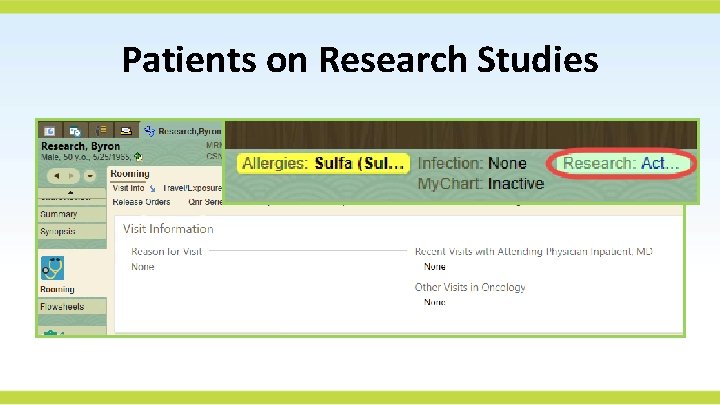
Patients on Research Studies

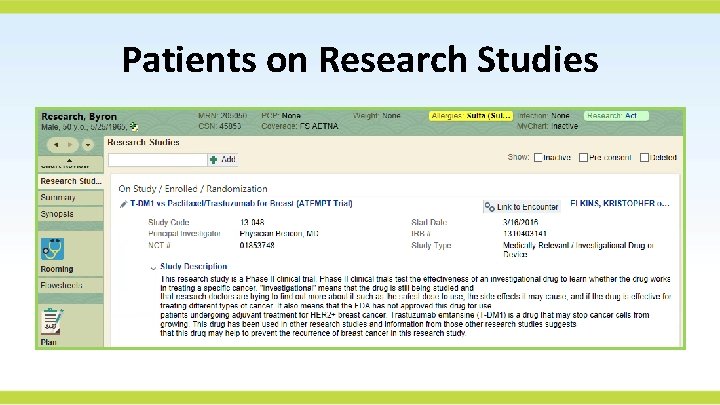
Patients on Research Studies

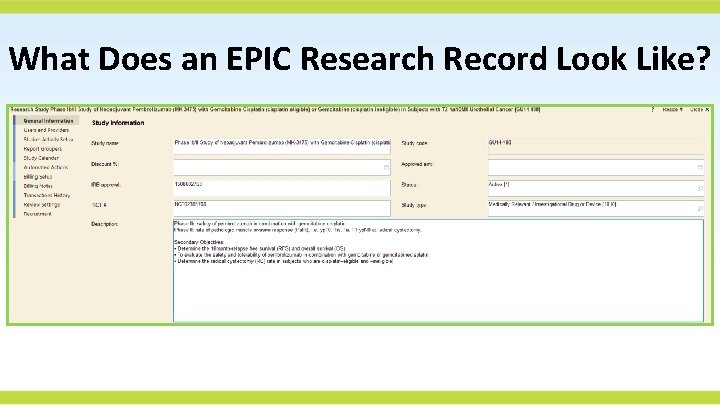
What Does an EPIC Research Record Look Like?

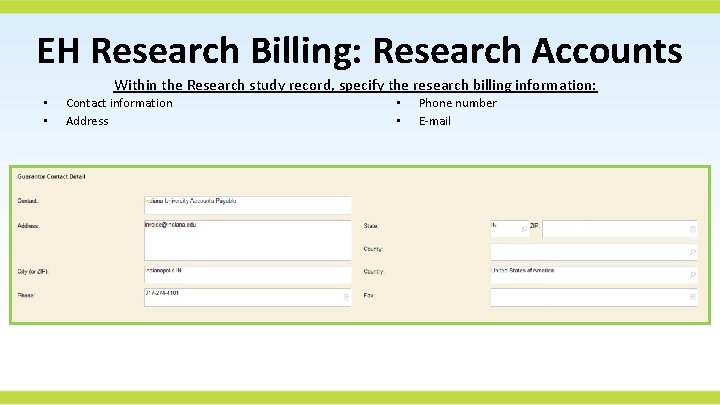
EH Research Billing: Research Accounts Within the Research study record, specify the research billing information: • • Contact information Address • • Phone number E-mail

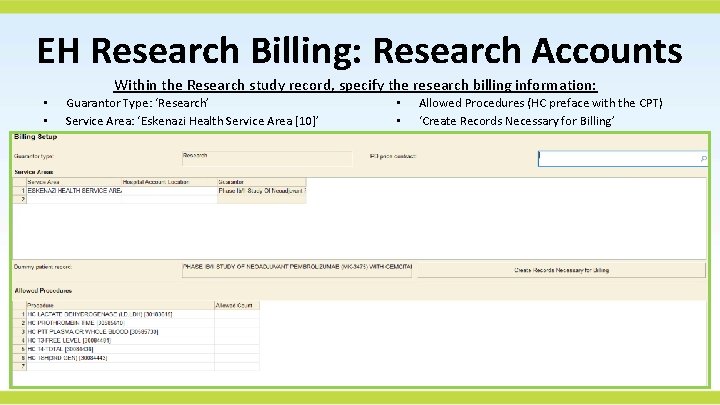
EH Research Billing: Research Accounts Within the Research study record, specify the research billing information: • • Guarantor Type: ‘Research’ Service Area: ‘Eskenazi Health Service Area [10]’ • • Allowed Procedures (HC preface with the CPT) ‘Create Records Necessary for Billing’

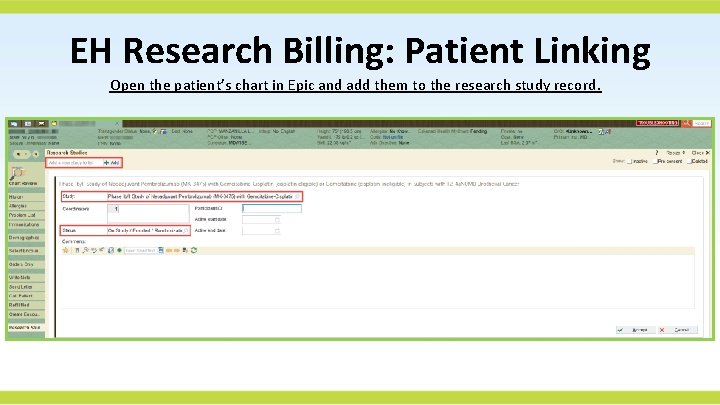
EH Research Billing: Patient Linking Open the patient’s chart in Epic and add them to the research study record.

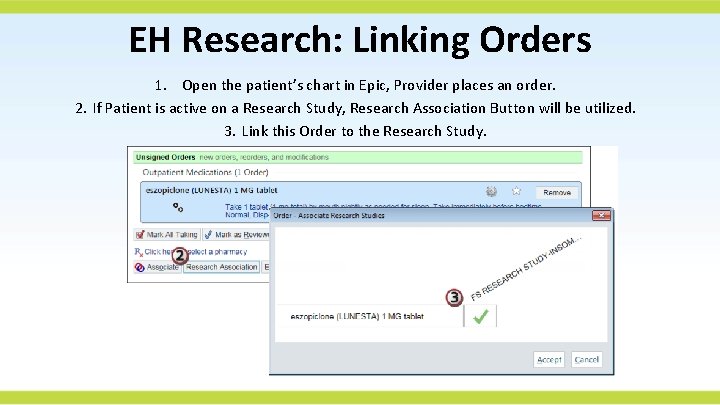
EH Research: Linking Orders 1. Open the patient’s chart in Epic, Provider places an order. 2. If Patient is active on a Research Study, Research Association Button will be utilized. 3. Link this Order to the Research Study.

EH Research Billing: Quick Reports

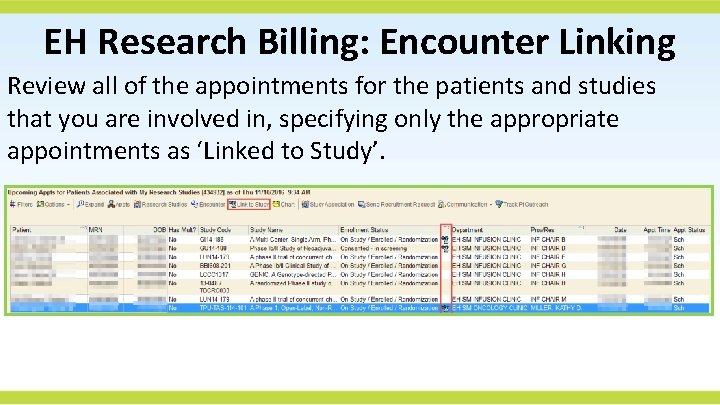
EH Research Billing: Encounter Linking Review all of the appointments for the patients and studies that you are involved in, specifying only the appropriate appointments as ‘Linked to Study’.

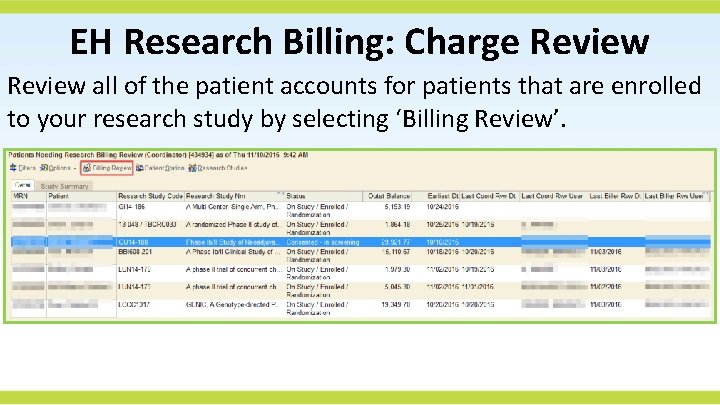
EH Research Billing: Charge Review all of the patient accounts for patients that are enrolled to your research study by selecting ‘Billing Review’.

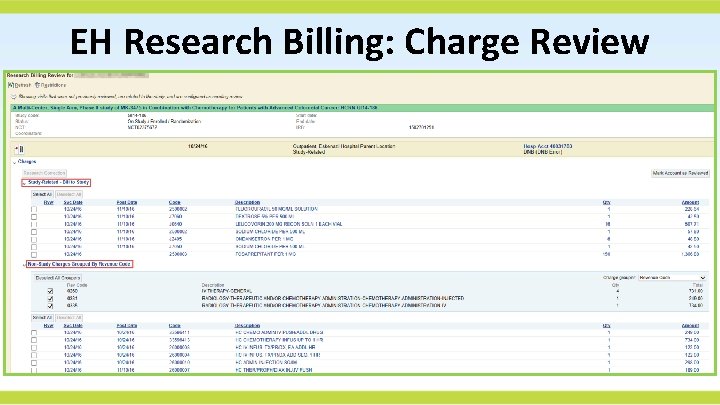
EH Research Billing: Charge Review

HOW TO CLOSE AN EH STUDY

Closing an Active Study • CLOSE the study at EH when: • • No longer conducting research at EH No longer completing procedures with EH patients (regardless of location) No longer accessing medical records for the purposes of research Regardless of closure w/ IU IRB • Email “Close” notification to: • Research 1@eskenazihealth. edu – ALL RESEARCH • Research. billing@eskenazihealth. edu – To close a Research Billing Account • CC the Principal Investigator as acknowledged • Reference the IU IRB Study Number and all applicable EH specific account numbers • You will receive a close out letter from EH to file with your study records

Don’t Forget! • All information/forms from today’s presentation is available on the IU Clinical Trials Office (CTO) website: https: //indianaclinicaltrialsoffice. iu. edu/investigators -study-coordinators/eskenazi-health/

Contact Information • • If you have questions regarding the overall process for the Eskenazi Health Research Application or any other general research questions, contact: Research 1@Eskenazi. Health. edu • If you have questions regarding Epic training and access, contact: Research. Training@Eskenazi. Health. edu • If you have questions regarding the Eskenazi Health credentialing process, contact: Suzanne. Maxwell@Eskenazi. Health. edu • • If you have any questions regarding Billing, contact: Research. Billing@Eskenazi. Health. edu

Many Thanks! • Many thanks to all Research Compliance Committee Members! • Bill Barnett • Kenneth Carlson • Kimberly Curry • Bob Davis • Kris Elkins • Debra Fawcett • Phyllis Garrison • Megan Gaunnac • Chanda Halderman • David Hass • Andrea Heid • Kim Howland • Colette Jackson • Gail Jenkins • Amy Little • Suzanne Maxwell • Melissa Materson • Lenora Maze • Nikki Mehdiyoun • Justin Morea • Margie Payne • Christopher Scott • Tammy Shoultz • Brian Smith • Amy Waltz • Shawn Wellman • Sylvia Wilcox • John Putz • Jennifer Hillstrom

Questions?
 Eskenazi epic
Eskenazi epic Homer's first epic poem
Homer's first epic poem What makes an epic an epic
What makes an epic an epic Overview of education in health care
Overview of education in health care Overview in research example
Overview in research example Go epic health
Go epic health Epic research module
Epic research module Role of community health nurse in occupational health
Role of community health nurse in occupational health National program related to child health and welfare
National program related to child health and welfare Ldh health standards
Ldh health standards Distinguish between education and health services
Distinguish between education and health services Whole health circle of health
Whole health circle of health Health and social care component 3
Health and social care component 3 Health maintenance and promotion
Health maintenance and promotion Pcb 3703c ucf
Pcb 3703c ucf Chapter 3 health wellness and health disparities
Chapter 3 health wellness and health disparities Health propaganda definition
Health propaganda definition Chapter 1 understanding health and wellness lesson 2
Chapter 1 understanding health and wellness lesson 2 Understanding health and wellness chapter 1
Understanding health and wellness chapter 1 Optimal health in each of the six components of health
Optimal health in each of the six components of health Australian primary health care research institute
Australian primary health care research institute Telethon institute for child health research
Telethon institute for child health research Health care systems research network
Health care systems research network Australian ehealth research centre
Australian ehealth research centre Community health nurse role
Community health nurse role Level 5 health and social care research project examples
Level 5 health and social care research project examples Pico question example
Pico question example Agency for health care research and quality
Agency for health care research and quality International institute of health management research
International institute of health management research Jenis penelitian kesehatan
Jenis penelitian kesehatan Commonwealth health research board
Commonwealth health research board Research report vs research proposal
Research report vs research proposal Research design vs research method
Research design vs research method Appendices example in research paper
Appendices example in research paper Design research meaning
Design research meaning What is applied research
What is applied research Qualitative research
Qualitative research Contrast applied research and basic research
Contrast applied research and basic research Sources of a research problem
Sources of a research problem Research instrument in experimental research
Research instrument in experimental research