RED 1 What are the propertiescharacteristics of metals






- Slides: 8

RED 1. What are the properties/characteristics of metals? 2. What are the properties/characteristics of nonmetals? 3. What are the properties/characteristics of metalloids? 4. What is a pure substance? What are the two types of pure substance? 5. What is a mixture? What are the two categories of mixtures? 6. What are the single particles in an element? compound? 7. What is matter? 1. malleable, ductile, high melting point, shiny, conducts electricity 2. Brittle, dull, does not conduct electricity 3. Both properties of metals and nonmetals 4. A substance that is only made of one type of particle elements and compounds 5. A substance that is made of two or more particles heterogenous and homogenous 6. element-atom compound-molecule 7. Anything that has mass and takes up space.

PURPLE 1. Given a sample of an unknown substance what steps would you use to determine its identity. 2. What do you create when you combine two atoms of elements? 3. What type of change separates a mixture? A compound? 4. Why can’t we ever find a sample of of an element by itself? 5. How would you separate a box of plastic pieces, aluminum pieces, and iron pieces? 6. What is the difference between a solute and a solvent? 7. You have a solution that has 5 parts KCl and 10 parts H 20 and a solution that is 15 parts KCL and 20 parts H 20. Which solution is more concentrated? How do you know? 1. 2. 3. 4. 5. 6. 7. Look at its characteristic properties: density, melting/freezing point, boiling/condensing point A compound Mixture-Physical Compound-Chemical Because they are constantly trying to become stable they are seeking other atoms to bond with. Use a magnet to remove the iron pieces, fill the container with water so the plastic pieces float to the top. Solute is what is added and dissolved Solvent is where a solute is added and is doing the dissolving. 15: 20 because there are more parts of solute in a given solvent.

ORANGE 1. How does crushing a solute increase its solubility? 2. Why is it easier to dissolve sugar in hot coffee, than in iced coffee? 3. How would you determine if a substance was a solution, suspension, or a colloid? 4. When two poisonous elements combine is the new compound poisonous? (Yes or No) Why? 5. What is the difference between a heterogeneous and homogenous mixture? 1. 2. More surface area The higher temperature causes the particles to move faster allowing it to dissolve faster. 3. Shine a flashlight, check to see if it settles out or not. 4. Not always, a compound may have different properties of the elements that combined together to form it. 5. Heterogeneous-appears to be different throughout Homogeneous-appears to be the same throughout

BLUE Identify the following as solution, suspension, or colloid? 1. Muddy Water Suspension 2. Salt Water Solution 3. Lemonade Solution 4. Gelatin Colloid 5. Milk Colloid 6. Snow Globe Suspension 7. Butter Colloid

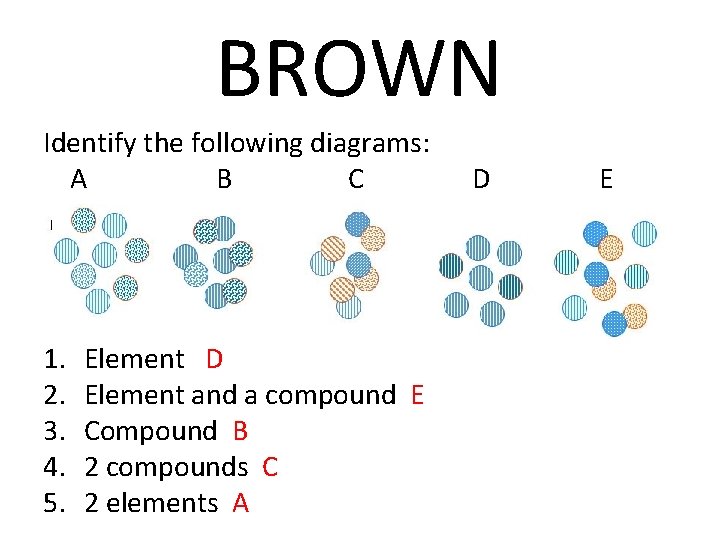
BROWN Identify the following diagrams: A B C 1. 2. 3. 4. 5. Element D Element and a compound E Compound B 2 compounds C 2 elements A D E

YELLOW Classify the following as a heterogeneous or homogeneous mixture. 1. Mixture that scatters light 2. Mixture easily filtered 3. Mixtures that does not scatter light 4. Mixture appears to be made of one substance. 5. Mixture that settles over time 6. Mixture made of small particles 7. Mixture made of larger particles 1. 2. 3. 4. 5. 6. 7. Heterogeneous Homogeneous Heterogeneous

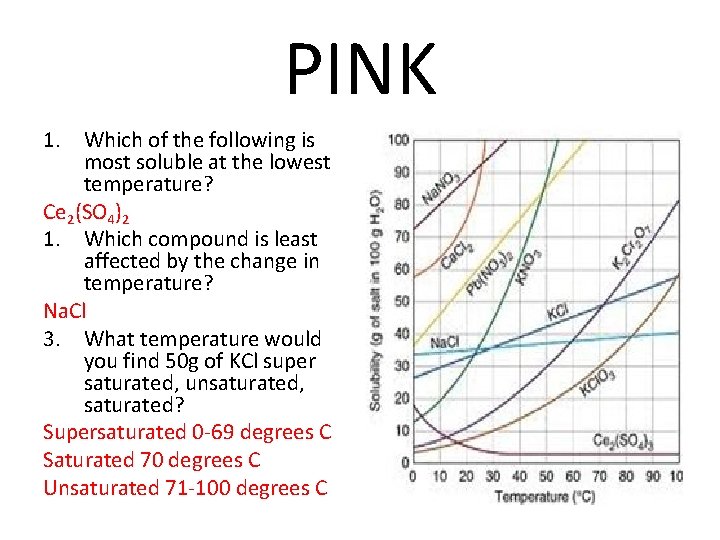
PINK 1. Which of the following is most soluble at the lowest temperature? Ce 2(SO 4)2 1. Which compound is least affected by the change in temperature? Na. Cl 3. What temperature would you find 50 g of KCl super saturated, unsaturated, saturated? Supersaturated 0 -69 degrees C Saturated 70 degrees C Unsaturated 71 -100 degrees C

GREEN 1. 2. 3. 4. 5. 6. 38 dag = _______hg 0. 8 dg = _______kg 5 m = ____cm 24 l = _____dl 100 C = _____ F 32 F = _____ C 1. 2. 3. 4. 5. 6. 3. 8 hg. 00008 kg 500 cm 250 liters 212 degrees F 0 degrees C