READING THE DOLLY PAPER PAPER CRITIQUE GUIDELINES I



















- Slides: 22

READING THE DOLLY PAPER & PAPER CRITIQUE GUIDELINES I am “Ewe”nique!!! Giles & Knight. News. Nature 2003 Viable offspring derived from fetal & adult mammalian cells. Wilmut et al. 1997. Nature.

TITLE & ABSTRACT INTRODUCTION RESULTS

METHODS RESULTS DISCUSSION REFERENCES

1. Reading the Title and Abstract What question are they asking? How did they ask it? Techniques used Are the techniques the right ones? What were their results? What would bolster these results? What were their conclusions? Are they the right conclusions? Why or why not? What else do they need? What would you do?

2. Reading the Introduction • Important background for people not in the same field • How the authors think their work fits into their field – The Big Picture – Why the question is being asked – Why certain techniques were chosen

3. Reading the Results One figure at a time: What is the smaller question being asked? A. What technique was used B. What results were obtained - what does the figure show? C. What conclusions were drawn from the results D. Are the conclusions appropriate? How does the result help to answer the question? Think: What else would I do? How?

4. Reading the Discussion Often a brief summary of the findings in the paper The experimenters’ conclusions about their material Are they right? Are they reaching? Think about the real conclusions of the data given What could convince you - think of “Future experiments” that need to be done and write them down!

5. Reading The Methods Section Extra details about experiments Provides other researchers with enough information to replicate experiments Often full of jargon and abbreviations, and targeted to other scientists in the same field Usually reference previous work - more trips to the library Why you should come to section!! Questions: ask your TAs!

Review Guidelines - Format • 12 point font • Standard (0. 5 in) margins • Single spaced • ONE PAGE ONLY - Anything on a second page will not be graded. - Brevity is key! • 10 Points Total

Scoring - The Quick Stuff Background: 1 point Stick to info given in paper - no extra research Goal of Experiment: 1 point What were they trying to do? Why - Hypothesis? Explanation of experiment: 1 point What did they involve (broad methods) and what techniques were employed? Interpretation of results: 1 point What do they mean? Do they support/argue against hypothesis? Correctness: 1 point Subjective point: 1 (grammar, spelling etc) Keep this section of your review brief (avoid wordiness!) and in your own words

Scoring - The IMPORTANT Bit CRITIQUE: 2 points GOOD: What did the researchers do right or wrong? What could they have done better? Be specific! BAD (0 points): “The researchers present evidence both for and against their hypothesis. They accept the hypothesis, but do not explain (not very clearly at least) why the “for” evidence is more significant than the “against” evidence. ” FUTURE EXPERIMENTS: 2 points GOOD: Design an additional experiment that addresses the deficiencies in the paper or provides corroborative evidence for the conclusions it draws. Be specific and creative! BAD (0 points): “Further work must be done to determine which of these models, if any, is the correct one. ”

Dolly Paper Example Critique Part D”ewe” Giles & Knight. News. Nature 2003

1. Background - 1 PT Embryonic nuclei rapidly lose ability to generate a tadpole as they age (Briggs & King, 1965) See Gilbert, chapter 4 Nuclei from adult keratinocytes can give rise to tadpoles, but not to adults. (Gurdon et al. , 1975) Nuclei from cultured embryonic cells induced into quiescence can give rise to lambs. (Wilmut 1996, 1997) Quiescence = cell inactivity (G 0) In your OWN words! Condense!!! *Only a few sentences*

2. Goal of experiment - 1 PT Hypothesis: “We suggested that inducing the donor cell to exit the growth phase causes changes in chromatin structure that facilitate reprogramming of gene expression and that development would be normal if nuclei are used from a variety of differentiated donor cells in similar regimes. ” • Wilmut et al. hypothesize mammalian nuclei can generate viable offspring if they are properly reprogrammed by being forced to exit from the cell cycle into G 0. Goal: “Here we investigate whether normal development to term is possible when donor cells derived from fetal or adult tissue are induced to exit the growth cycle and enter the G 0 phase of the cell cycle before nuclear transfer. ” • In this study, Wilmut et al. test the ability of nuclei from cells at different stages of development to generate a complete lamb.

3. Explanation of Experiments - 1 PT Three different cell types were used as nuclear donors: Embryonic cells were cultured from a 9 day old Poll Dorset embryo Fetal cells were derived from a 26 day old Black welsh Mountain fetus. Mammary gland cells were obtained from a 6 yr old Finn Dorset ewe. Donor cells were checked for the correct number of chromosomes (54) and forced to exit the cell cycle through serum starvation. In the presence of serum (growth factors), cells progress through the cell cycle and divide rapidly In the absence of serum (starvation), cells can survive by entering the G 0 phase and remaining quiescent Nuclear Transfer The cloning technique.

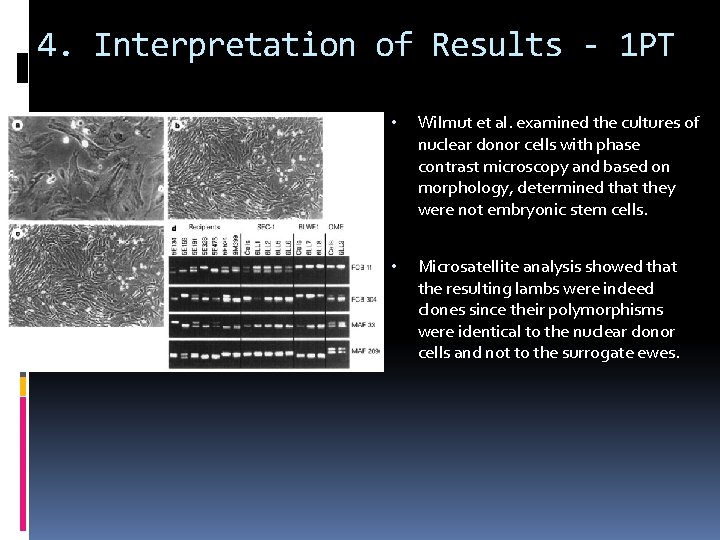
4. Interpretation of Results - 1 PT • Wilmut et al. examined the cultures of nuclear donor cells with phase contrast microscopy and based on morphology, determined that they were not embryonic stem cells. • Microsatellite analysis showed that the resulting lambs were indeed clones since their polymorphisms were identical to the nuclear donor cells and not to the surrogate ewes.

5. Critique - 2 PTS Have they supported their hypothesis? (Read it carefully) “We suggested that inducing the donor cell to exit the growth phase causes changes in chromatin structure that facilitate reprogramming of gene expression & that development would be normal if nuclei are used from a variety of differentiated donor cells in similar regimes. ”

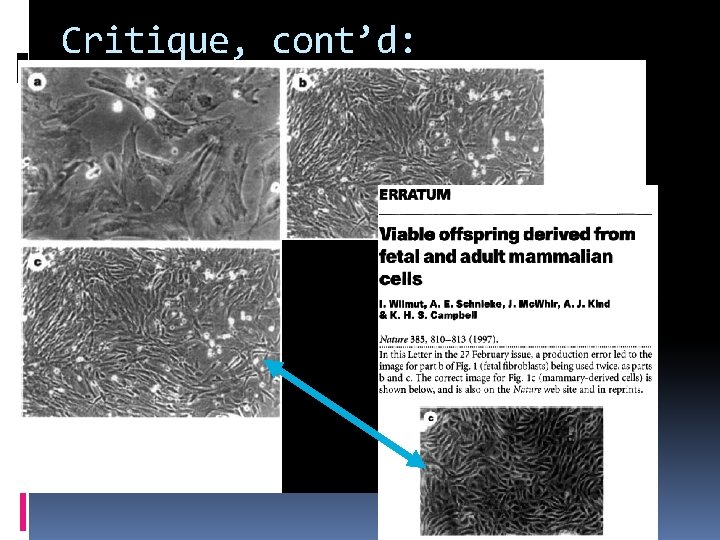
Critique, cont’d: Look at figures closely!

Critique, cont’d:

6. Future Experiments - 2 PTS Mammary epithelium cells can divide quickly and the source they used was a mixed population from a pregnant ewe. Test the ability of a differentiated cell that is unlikely to divide quickly such as nuclei from neurons. If cell cycle is the only thing that really matters you should be able to get embryos.

These are not the result of nuclear transfer! http: //www. nerdie. com/animalcloning. html

Office Hour Information Jon Valencia jev@caltech. edu Monday 5 -6 PM (the following week) Thursday 5 -6 PM (the current week) Building & Room: Kerckhoff 017