REACH Regulation An Overview Contents Understanding REACH How














- Slides: 17

REACH Regulation An Overview

Contents • Understanding REACH • How does REACH work • Obligations under REACH • What do you manufacture/import • Registration – Substances to be registered • Data sharing • Duty to communicate

Understanding REACH is a regulation of the EU, adopted to improve the protection of human health and the environment from the risks that can be posed by chemicals, while enhancing the competitiveness of the EU chemicals industry. It also promotes alternative methods for the hazard assessment of substances in order to reduce the number of tests on animals.

Understanding REACH Cont. In principle, REACH applies to all chemical substances; not only those used in industrial processes but also in our day-to-day lives, for example in cleaning products, paints as well as in articles such as clothes, furniture and electrical appliances. Therefore, the regulation has an impact on most companies across the EU.

Understanding REACH Cont. REACH places the burden of proof on companies. To comply with the regulation, companies must identify and manage the risks linked to the substances they manufacture and market in the EU. They have to demonstrate to ECHA how the substance can be safely used, and they must communicate the risk management measures to the users.

Understanding REACH Cont. • If the risks cannot be managed, authorities can restrict the use of substances in different ways. In the long run, the most hazardous substances should be substituted with less hazardous ones. • REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals. It entered into force on 1 June 2007.

How does REACH work • REACH establishes procedures for collecting and assessing information on the properties and hazards of substances. • Companies need to register their substances and to do this they need to work together with other companies who are registering the same substance. • ECHA receives and evaluates individual registrations for their compliance, and the EU Member States evaluate selected substances to clarify initial concerns for human health or for the environment. Authorities and ECHA's scientific committees assess whether the risks of substances can be managed. • Authorities can ban hazardous substances if their risks are unmanageable. They can also decide to restrict a use or make it subject to a prior authorisation.

Obligations under REACH • Different obligations are specified under REACH • Depending on the role in the supply chain • Manufacturer • Importer • Only representative • Downstream user • First step is to identify what is your role and obligations under REACH

What do you manufacture/ import • Different obligations depending whether: Substance – only substances have to be registered Preparation – when contained in a preparation each individual substance needs to be registered. Substances that have been registered by the manufacturer/importer and that are being mixed in to a preparation by a downstream user, do not need to be registered again by the latter. Article – individual substances in articles are also potential for registration

Registration • Companies have the responsibility of collecting information on the properties and the uses of substances that they manufacture/import at or above 1 tonne/year. • Companies also have to make an assessment of the hazards and potential risks presented by the substance. • This info is communicated to ECHA through a registration dossier containing the hazard information and, where relevant, an assessment of the risks that the use of the substance may pose and how these risks should be controlled. • Registration applies to substances on their own, substances in mixtures and certain cases of substances in articles.

Registration Cont. • Chemical substances that are already regulated by other legislations such as medicines, or radioactive substances are partially or completely exempted from REACH requirements. • Registration is based on the "one substance, one registration" principle. This means that manufacturers and importers of the same substance have the obligation to submit their registration jointly. • For substance registration a fee is usually charged.

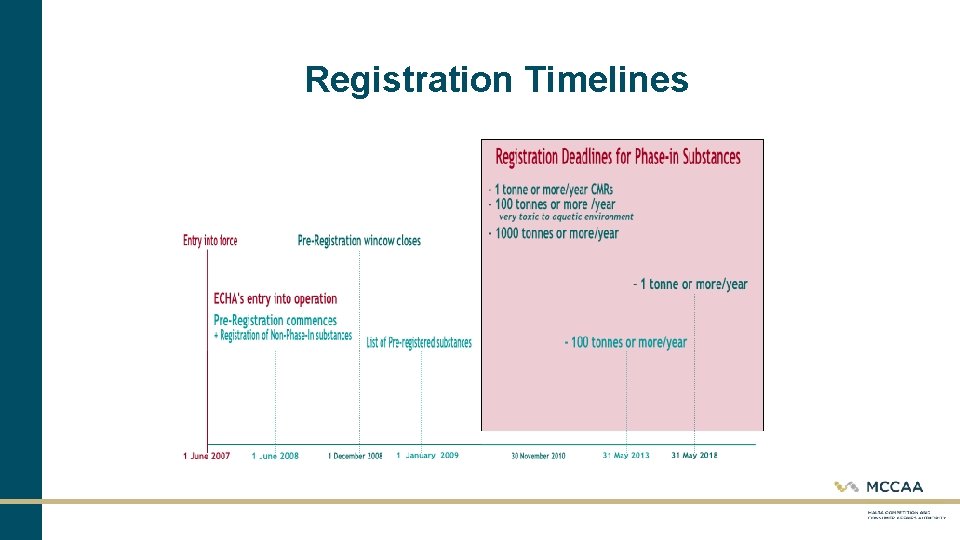
Registration – Substances to be registered • The legislation allowed for a transitional regime for substances which, under certain conditions, were already manufactured or placed on the market before REACH's entry into force. • Substances fulfilling at least one of the following criteria may be considered as phase-in substances: Substances listed in the European Inventory of Existing Commercial Chemical Substances (EINECS) Substances that have been manufactured in the EU (including the countries that joined on 1 January 2007) but have not been placed on the EU market after 1 June 1992 Substances that qualify as "no-longer polymer''

Registration Timelines

Registration – Substances to be registered Cont. • All substances that do not fulfil any of the criteria for phase-in substances are considered as non-phase-in substances. • Normally, non-phase-in substances have not been manufactured, placed on the market or used in the EU before 1 June 2008. • Potential manufacturers and importers of non-phase-in substances have to submit an inquiry to ECHA and subsequently register the substance in accordance with REACH before they can manufacture or import the substance.

Data sharing • Data collected through vertebrate animal testing must be shared, against payment. • Substance Information Exchange Forum (SIEF) (for pre-registered substances) • prior to registration • before testing is carried out • Inquiry process – for substances which have not been preregistered • potential registrant inquires from the Agency

Duty to communicate • The registrant communicates with his Downstream Users so as to be able to prepare the registration dossier. • In particular the registrant will need information about DUs uses and the risk management measures they have already put in place • Provide a Safety Data Sheet (SDS) to customers • The final Exposure Scenario developed for identified uses as part of the Chemical Safety Assessment has to be communicated to the registrant’s customers as an annex to the SDS as this provides instructions of risk management measures that should be in place in order to ensure adequate control of risks. • The SDS will have to be updated with the new information required by REACH for the first supply of the substance or preparation as soon as this information is required by the different title of REACH.

Thank you for your attention
 Contents of audit planning memorandum
Contents of audit planning memorandum Hamlet historical context
Hamlet historical context Contents provider
Contents provider Mediastinum contents
Mediastinum contents Contents background
Contents background Femoral triangle applied anatomy
Femoral triangle applied anatomy A 'contents' key is required
A 'contents' key is required Fresh frozen plasma transfusion time
Fresh frozen plasma transfusion time Science notebook table of contents
Science notebook table of contents Maudie's nutrition
Maudie's nutrition Swot for event planning
Swot for event planning Nature of prospectus
Nature of prospectus Ark
Ark Table of contents case study
Table of contents case study Carotid sheath contents
Carotid sheath contents Inguinal pouch
Inguinal pouch Trial master file contents
Trial master file contents What is the role of gastric juice
What is the role of gastric juice