OASIS Electronic Trial Master File Standard Technical Committee




- Slides: 23

OASIS Electronic Trial Master File Standard Technical Committee Metadata Component Content Model Component February 17, 2014 9: 00 – 10: 00 AM PST

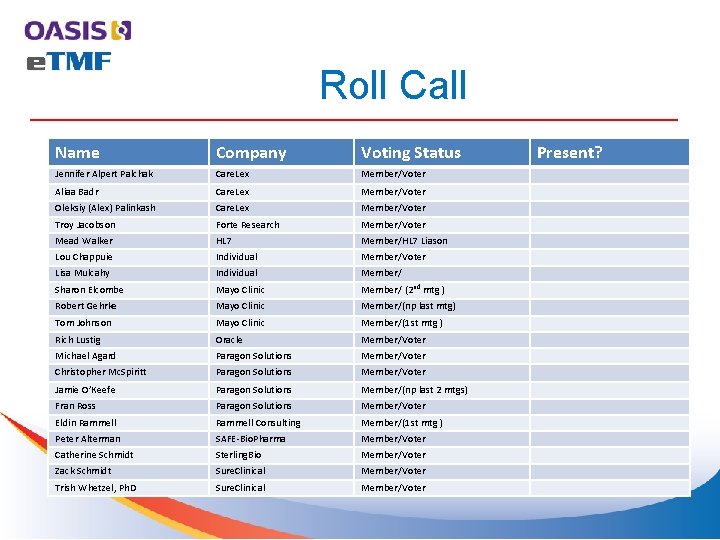
Agenda Topic 9: 00 -9: 05 Call to Order & Roll Call 9: 05 -9: 10 Approval of Minutes Presenter Zack Schmidt https: //www. oasisopen. org/committees/documents. php? wg_abbrev=etmf All 9: 10 -9: 15 OASIS policy review: member/observer roles Chet Ensign 9: 10 -9: 20 Outreach Subcommittee - All Jennifer Alpert 9: 20 -9: 30 Tech pres – Metadata, Content Model Components Z. Schmidt 9: 30 -9: 50 Tech Discussion – Content Classification Layer All 9: 50 -9: 55 New Business All 9: 55 -10: 00 Next meeting agenda / Date Z. Schmidt 2

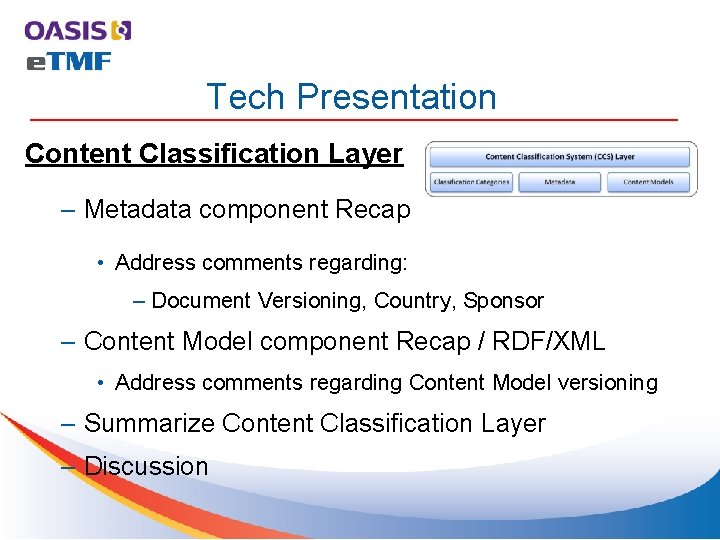
Roll Call Name Company Voting Status Jennifer Alpert Palchak Care. Lex Member/Voter Aliaa Badr Care. Lex Member/Voter Oleksiy (Alex) Palinkash Care. Lex Member/Voter Troy Jacobson Forte Research Member/Voter Mead Walker HL 7 Member/HL 7 Liason Lou Chappuie Individual Member/Voter Lisa Mulcahy Individual Member/ Sharon Elcombe Mayo Clinic Member/ (2 nd mtg ) Robert Gehrke Mayo Clinic Member/(np last mtg) Tom Johnson Mayo Clinic Member/(1 st mtg ) Rich Lustig Oracle Member/Voter Michael Agard Paragon Solutions Member/Voter Christopher Mc. Spiritt Paragon Solutions Member/Voter Jamie O’Keefe Paragon Solutions Member/(np last 2 mtgs) Fran Ross Paragon Solutions Member/Voter Eldin Rammell Consulting Member/(1 st mtg ) Peter Alterman SAFE-Bio. Pharma Member/Voter Catherine Schmidt Sterling. Bio Member/Voter Zack Schmidt Sure. Clinical Member/Voter Trish Whetzel, Ph. D Sure. Clinical Member/Voter Present?

Meeting Etiquette • Announce your name prior to making comments or suggestions • Keep your phone on mute when not speaking (#6) • Do not put your phone on hold – Hang up and dial in again when finished with your other call – Hold = Elevator Music = very frustrated speakers and participants • Meetings will be recorded and posted – Another reason to keep your phone on mute when not speaking! • Use the join. me “Chat” feature for questions / comments / Votes • NOTE: We will follow Robert’s Rules of Order This meeting is being recorded and minutes will be posted on TC page after the meeting 4 From e. TMF Std TC to Participants: Hi everyone: remember to keep your phone on mute

Outreach Subcommittee • Status – New Members: – Outreach Activity summary / Milestones – Joined: Tom Johnson, Sharon Elcombe /Mayo Clinic – In Progress: Shire – Deliverable – Summary Industry outreach / Comments report

Tech Presentation Content Classification Layer – Metadata component Recap • Address comments regarding: – Document Versioning, Country, Sponsor – Content Model component Recap / RDF/XML • Address comments regarding Content Model versioning – Summarize Content Classification Layer – Discussion

Metadata Component - Recap – Metadata Component: • Metadata (‘Tags’) – Characterizes content – Allows users to precisely search for information, create reports, share data online – Use of standards-based terms is critical for interoperability between systems

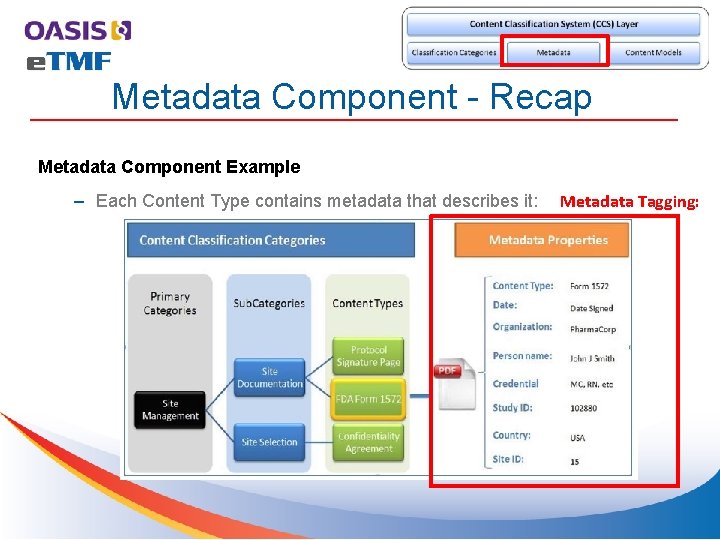
Metadata Component - Recap Metadata Component Example – Each Content Type contains metadata that describes it: Metadata Tagging:

Core Metadata – Document Version Numbering • Based on comments re: Doc Version support, a new metadata term is proposed: Document Version (applies to e. TMF Document or Content Item) • Based on NCI/CDISC/FDA/HL 7/BRIDG term definitions: – Per NCI/NIH/BRIDG: a ‘Representation of a particular edition or snapshot of a document as it exists at a particular point in time. ’ – NCI Code C 93484, NCI Code C 93816 – Follows industry standard ‘Major. Minor’ numbering: » Major =1. 0, Minor = 1. 1 • Document Version management is an application-specific / implementation specific task

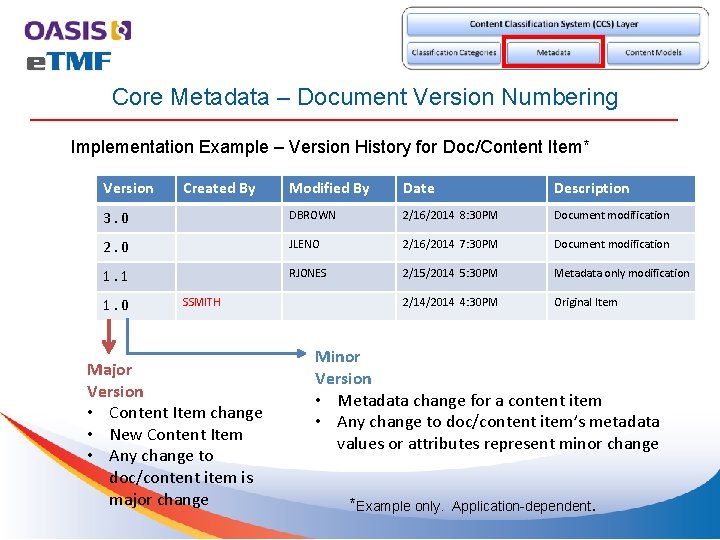
Core Metadata – Document Version Numbering Implementation Example – Version History for Doc/Content Item* Version Modified By Date Description 3. 0 DBROWN 2/16/2014 8: 30 PM Document modification 2. 0 JLENO 2/16/2014 7: 30 PM Document modification 1. 1 RJONES 2/15/2014 5: 30 PM Metadata only modification 2/14/2014 4: 30 PM Original Item 1. 0 Created By SSMITH Major Version • Content Item change • New Content Item • Any change to doc/content item is major change Minor Version • Metadata change for a content item • Any change to doc/content item’s metadata values or attributes represent minor change *Example only. Application-dependent.

Core Metadata – Document Version Numbering Policy Document Version number text formatting In the e. TMF Standard, the document version text values follow the same formatting that is familiar and commonly implemented in software and in other health science standards: Major Version. Minor Version numbering text are integer values separated by a period, without leading zeros. There can be a new Major version every time the document/content item changes. There can be a new Minor version every time the metadata changes. Version Numbering Policies (based on NCI/CDISC/FDA/BRIDG def: C 93816) Within e. TMF archives, document / content item version management shall be application specific to provide for application flexibility. However, for consistent content item exchange, version number text formatting should be implemented using e. TMF document version numbering policies: Each document Major version number is an integer starting at '1' and incrementing by 1. The first instance or original document should always be valued as '1'. The version number value must be incremented by one when a document is replaced, but can also be incremented more often to meet application specific requirements. Different versions of the same document belong to the same Content Type group. The document Minor version number would be an integer starting at ‘ 0' and incrementing by 1. The first instance of an original document with no minor version should always be valued as ‘ 1. 0’, where ‘ 0‘ indicates that no minor version exists. Documents with a change to the metadata values would require a minor version. The first minor version for a 1. 0 document would be indicated as 1. 1. Successive changes to any of the document’s metadata would increment the Minor version by 1, for example 1. 2 indicates major version 1 and minor version 2. The Minor version number value must be incremented by one when a document’s metadata is changed, but can also be incremented more often to meet application specific requirements.

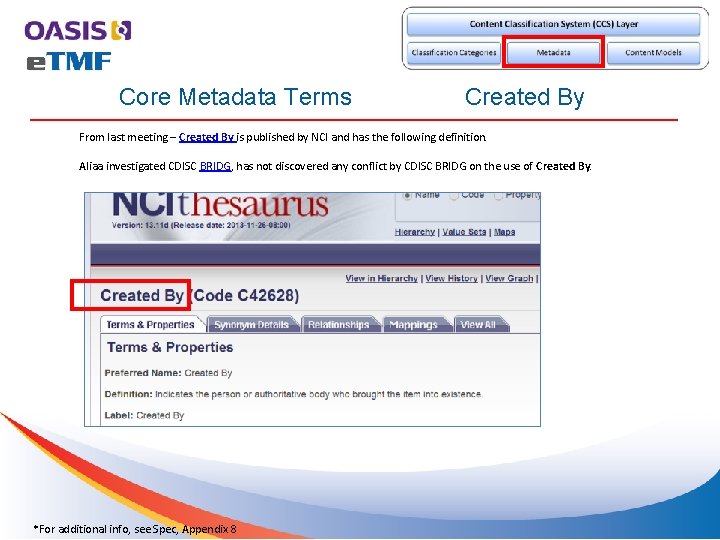
Core Metadata Terms Created By From last meeting – Created By is published by NCI and has the following definition. Aliaa investigated CDISC BRIDG, has not discovered any conflict by CDISC BRIDG on the use of Created By. *For additional info, see Spec, Appendix 8

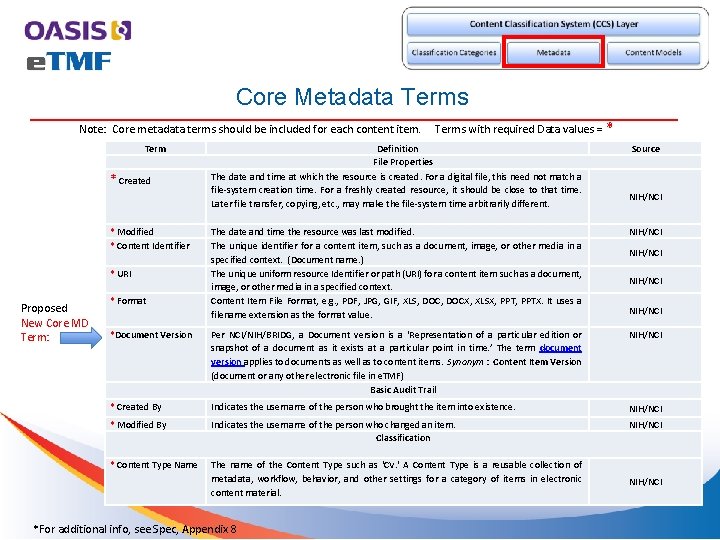
Core Metadata Terms Note: Core metadata terms should be included for each content item. Term * Created * Modified * Content Identifier Definition File Properties The date and time at which the resource is created. For a digital file, this need not match a file-system creation time. For a freshly created resource, it should be close to that time. Later file transfer, copying, etc. , may make the file-system time arbitrarily different. * Source NIH/NCI The date and time the resource was last modified. The unique identifier for a content item, such as a document, image, or other media in a specified context. (Document name. ) The unique uniform resource Identifier or path (URI) for a content item such as a document, image, or other media in a specified context. Content Item File Format, e. g. , PDF, JPG, GIF, XLS, DOCX, XLSX, PPTX. It uses a filename extension as the format value. NIH/NCI *Document Version Per NCI/NIH/BRIDG, a Document version is a ‘Representation of a particular edition or snapshot of a document as it exists at a particular point in time. ’ The term document version applies to documents as well as to content items. Synonym : Content Item Version (document or any other electronic file in e. TMF) Basic Audit Trail NIH/NCI * Created By Indicates the username of the person who brought the item into existence. NIH/NCI * Modified By Indicates the username of the person who changed an item. Classification NIH/NCI * Content Type Name The name of the Content Type such as 'CV. ' A Content Type is a reusable collection of metadata, workflow, behavior, and other settings for a category of items in electronic content material. * URI Proposed New Core MD Term: Terms with required Data values = * Format *For additional info, see Spec, Appendix 8 NIH/NCI

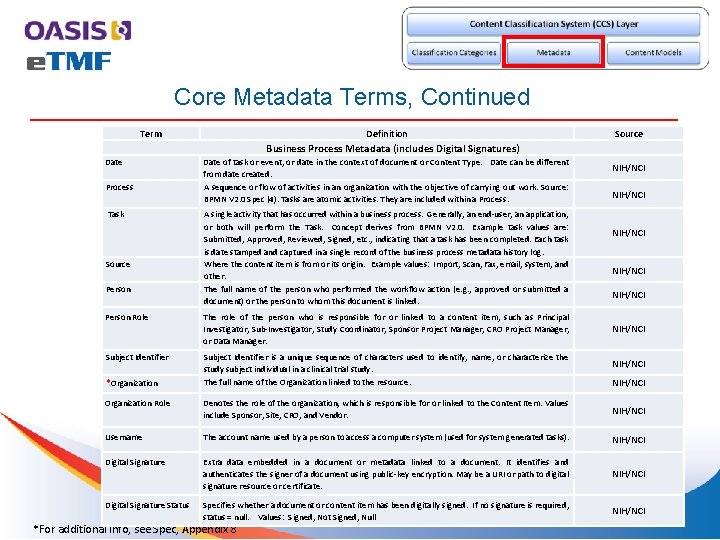
Core Metadata Terms, Continued Term Definition Source Business Process Metadata (includes Digital Signatures) Date Process Task Source Person Role Subject Identifier *Organization Date of task or event, or date in the context of document or Content Type. Date can be different from date created. A sequence or flow of activities in an organization with the objective of carrying out work. Source: BPMN V 2. 0 Spec (4). Tasks are atomic activities. They are included within a Process. A single activity that has occurred within a business process. Generally, an end-user, an application, or both will perform the Task. Concept derives from BPMN V 2. 0. Example task values are: Submitted, Approved, Reviewed, Signed, etc. , indicating that a task has been completed. Each task is date stamped and captured in a single record of the business process metadata history log. Where the content item is from or its origin. Example values: Import, Scan, Fax, email, system, and other. The full name of the person who performed the workflow action (e. g. , approved or submitted a document) or the person to whom this document is linked. The role of the person who is responsible for or linked to a content item, such as Principal Investigator, Sub-Investigator, Study Coordinator, Sponsor Project Manager, CRO Project Manager, or Data Manager. Subject Identifier is a unique sequence of characters used to identify, name, or characterize the study subject individual in a clinical trial study. The full name of the Organization linked to the resource. NIH/NCI NIH/NCI Organization Role Denotes the role of the organization, which is responsible for or linked to the Content Item. Values include Sponsor, Site, CRO, and Vendor. NIH/NCI Username The account name used by a person to access a computer system (used for system generated tasks). NIH/NCI Digital Signature Extra data embedded in a document or metadata linked to a document. It identifies and authenticates the signer of a document using public-key encryption. May be a URI or path to digital signature resource or certificate. NIH/NCI Specifies whether a document or content item has been digitally signed. If no signature is required, status = null. Values: Signed, Not Signed, Null NIH/NCI Digital Signature Status *For additional info, see Spec, Appendix 8

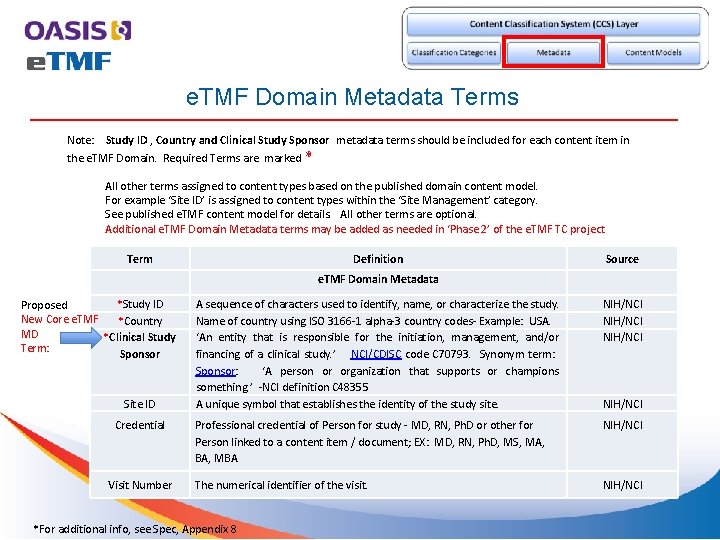
e. TMF Domain Metadata Terms Note: Study ID , Country and Clinical Study Sponsor metadata terms should be included for each content item in the e. TMF Domain. Required Terms are marked * All other terms assigned to content types based on the published domain content model. For example ‘Site ID’ is assigned to content types within the ‘Site Management’ category. See published e. TMF content model for details. All other terms are optional. Additional e. TMF Domain Metadata terms may be added as needed in ‘Phase 2’ of the e. TMF TC project Term Definition Source e. TMF Domain Metadata *Study ID Proposed New Core e. TMF *Country MD *Clinical Study Term: Sponsor Site ID Credential Visit Number A sequence of characters used to identify, name, or characterize the study. Name of country using ISO 3166 -1 alpha-3 country codes- Example: USA. ‘An entity that is responsible for the initiation, management, and/or financing of a clinical study. ’ NCI/CDISC code C 70793. Synonym term: Sponsor: ‘A person or organization that supports or champions something. ’ -NCI definition C 48355 A unique symbol that establishes the identity of the study site. NIH/NCI Professional credential of Person for study - MD, RN, Ph. D or other for Person linked to a content item / document; EX: MD, RN, Ph. D, MS, MA, BA, MBA NIH/NCI The numerical identifier of the visit. NIH/NCI *For additional info, see Spec, Appendix 8 NIH/NCI

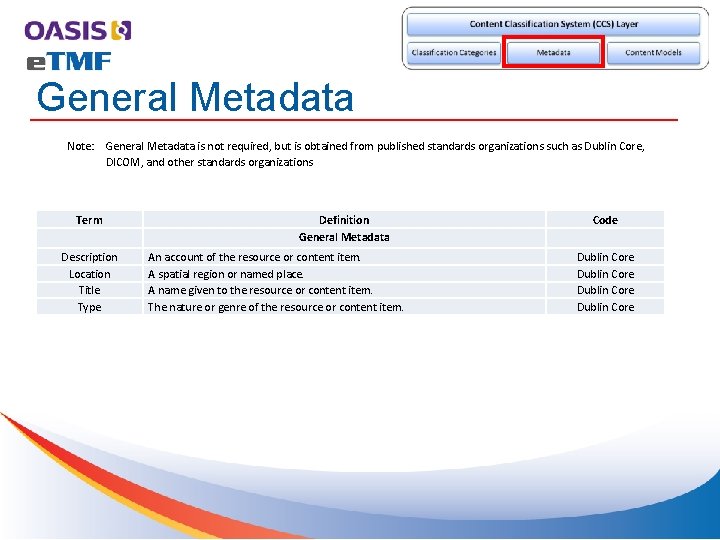
General Metadata Note: General Metadata is not required, but is obtained from published standards organizations such as Dublin Core, DICOM, and other standards organizations Term Description Location Title Type Definition General Metadata An account of the resource or content item. A spatial region or named place. A name given to the resource or content item. The nature or genre of the resource or content item. Code Dublin Core

Content Models • Recap on Content Models – What and Why – Content Model Format / Exchange – How Used • Content Model Versioning under W 3 C OWL/RDF/XML

Content Models What are Content Models (CM): • Represent content classifications, relationships, metadata in a semantic web taxonomy or ‘Ontology’ • CM’s are created using the W 3 C OWL 2 language and RDF/XML Why • Semantic web allows seamless sharing, linking of data across networks • Industry moving to Semantic web: – CDISC/FDA/PHuse project – HL 7, NIH/NCI, many more Recap: What and Why

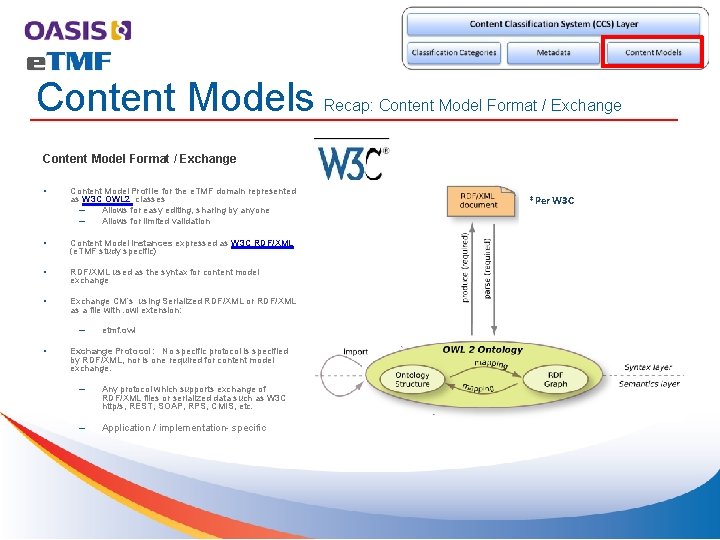
Content Models Recap: Content Model Format / Exchange • Content Model Profile for the e. TMF domain represented as W 3 C OWL 2 classes – Allows for easy editing, sharing by anyone – Allows for limited validation • Content Model Instances expressed as W 3 C RDF/XML (e. TMF study specific) • RDF/XML used as the syntax for content model exchange • Exchange CM’s using Serialized RDF/XML or RDF/XML as a file with. owl extension: – • etmf. owl Exchange Protocol: No specific protocol is specified by RDF/XML, nor is one required for content model exchange. – Any protocol which supports exchange of RDF/XML files or serialized data such as W 3 C http/s, REST, SOAP, RPS, CMIS, etc. – Application / implementation- specific *Per W 3 C

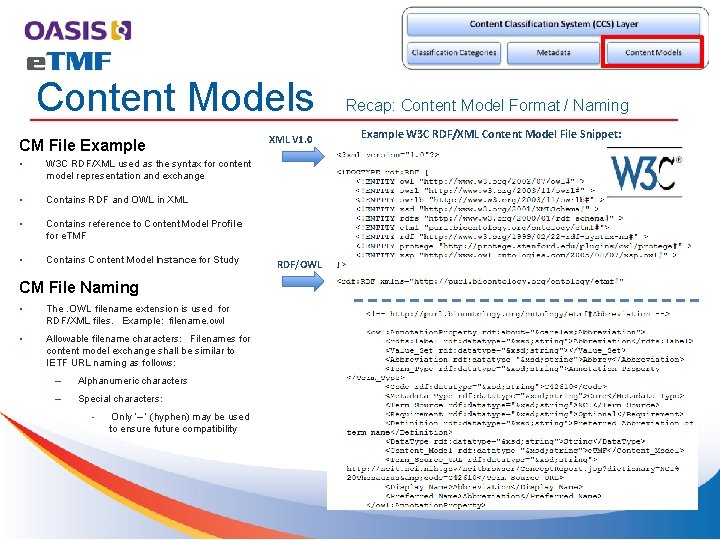
Content Models CM File Example • W 3 C RDF/XML used as the syntax for content model representation and exchange • Contains RDF and OWL in XML • Contains reference to Content Model Profile for e. TMF • Contains Content Model Instance for Study CM File Naming • The. OWL filename extension is used for RDF/XML files. Example: filename. owl • Allowable filename characters: Filenames for content model exchange shall be similar to IETF URL naming as follows: – Alphanumeric characters – Special characters: • Only ‘– ’ (hyphen) may be used to ensure future compatibility XML V 1. 0 RDF/OWL Recap: Content Model Format / Naming Example W 3 C RDF/XML Content Model File Snippet:

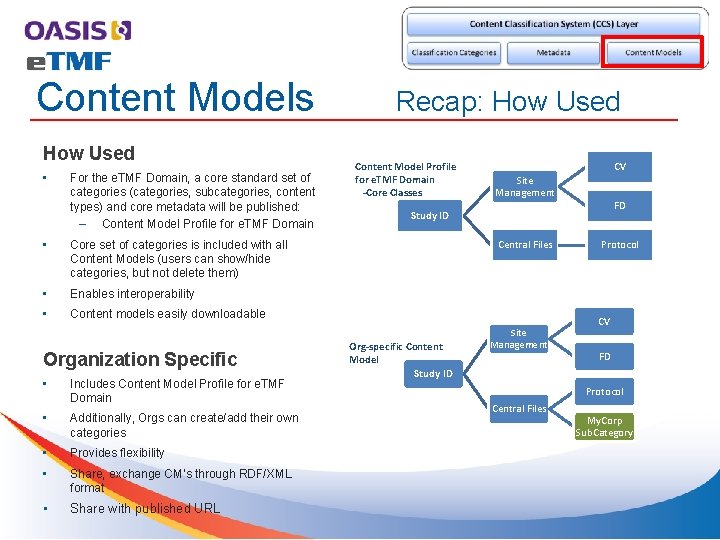
Content Models How Used • For the e. TMF Domain, a core standard set of categories (categories, subcategories, content types) and core metadata will be published: – Content Model Profile for e. TMF Domain • Core set of categories is included with all Content Models (users can show/hide categories, but not delete them) • Enables interoperability • Content models easily downloadable Organization Specific • Includes Content Model Profile for e. TMF Domain • Additionally, Orgs can create/add their own categories • Provides flexibility • Share, exchange CM’s through RDF/XML format • Share with published URL Recap: How Used Content Model Profile for e. TMF Domain -Core Classes CV Site Management FD Study ID Central Files Org-specific Content Model Study ID Site Management Protocol CV FD Protocol Central Files My. Corp Sub. Category

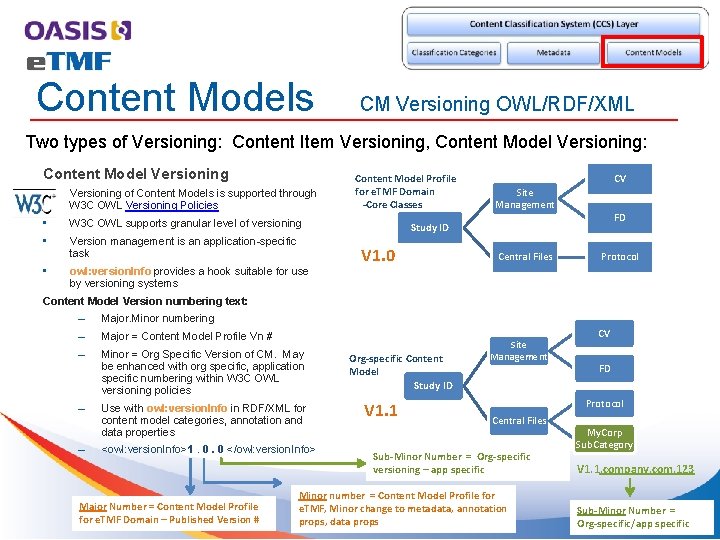
Content Models CM Versioning OWL/RDF/XML Two types of Versioning: Content Item Versioning, Content Model Versioning: Content Model Versioning • Versioning of Content Models is supported through W 3 C OWL Versioning Policies • W 3 C OWL supports granular level of versioning • Version management is an application-specific task • owl: version. Info provides a hook suitable for use by versioning systems Content Model Profile for e. TMF Domain -Core Classes CV Site Management FD Study ID V 1. 0 Central Files Protocol Content Model Version numbering text: – Major. Minor numbering – Major = Content Model Profile Vn # – Minor = Org Specific Version of CM. May be enhanced with org specific, application specific numbering within W 3 C OWL versioning policies – – Use with owl: version. Info in RDF/XML for content model categories, annotation and data properties <owl: version. Info>1. 0. 0 </owl: version. Info> Major Number = Content Model Profile for e. TMF Domain – Published Version # Org-specific Content Model Study ID V 1. 1 Site Management CV FD Protocol Central Files Sub-Minor Number = Org-specific versioning – app specific Minor number = Content Model Profile for e. TMF, Minor change to metadata, annotation props, data props My. Corp Sub. Category V 1. 1. company. com. 123 Sub-Minor Number = Org-specific/app specific

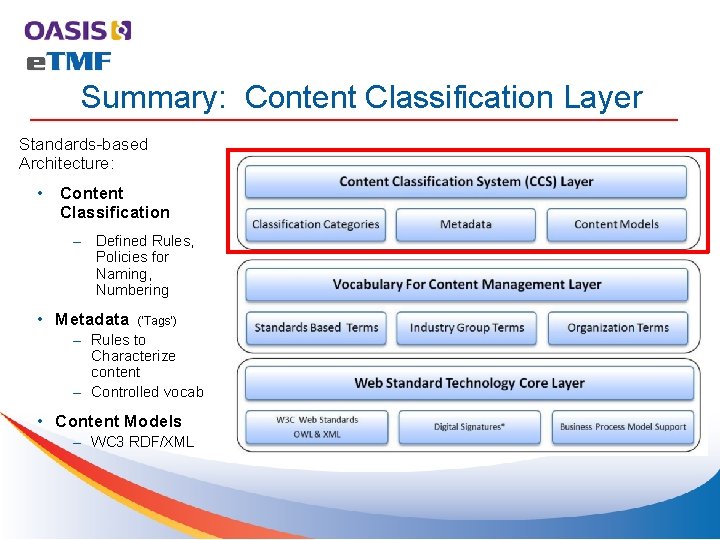
Summary: Content Classification Layer Standards-based Architecture: • Content Classification – Defined Rules, Policies for Naming, Numbering • Metadata (‘Tags’) – Rules to Characterize content – Controlled vocab • Content Models – WC 3 RDF/XML