Rapid Diagnostic Tests for Syphilis A Preliminary Review


- Slides: 17

Rapid Diagnostic Tests for Syphilis A Preliminary Review of the U. S. Clinical Data M Sutton, S Zackery, C Ciesielski, M Zajackowski, M Santana, C Langley, L Bernard, V Pope, M Fears, R Johnson, L Markowitz

Background n Syphilis is diagnosed in the U. S. by a nontreponemal screening test and a treponemal confirmatory test. q q n on-site RPR plus offsite treponemal test, OR RPR and treponemal tests are sent offsite (turn-around time up to 1 week or more). Rapid immunochromatographic (ICS) diagnostic tests: q treponemal-based tests q can provide results in 15 minutes q may potentially be of use in non-traditional settings n Rapid ICS tests for syphilis are not FDA-cleared for use in the US. n A U. S. based study was conceived to evaluate these rapid diagnostics for syphilis.

Multi-site study of rapid diagnostic syphilis assays of persons attending STD clinics in high syphilis morbidity areas n Cross-sectional study n 3 ICS strips included vary by which Treponema pallidum antigens are present on the test band region n Two U. S. sites are currently evaluating the performance of 3 rapid diagnostic strips

ICS Strip

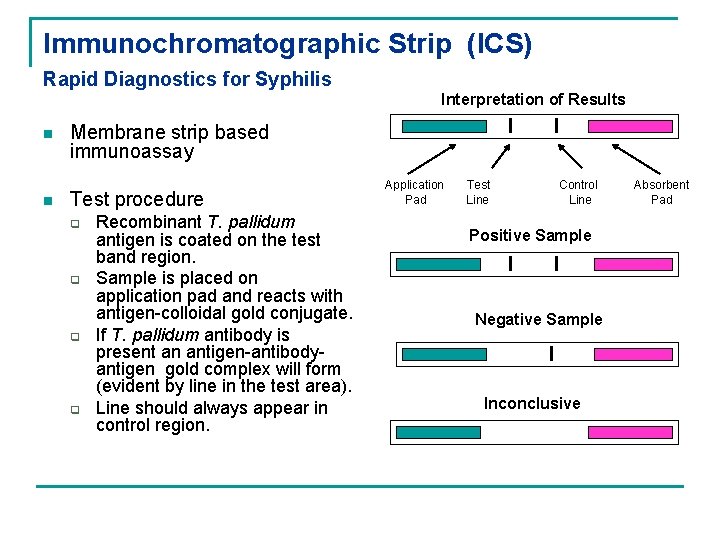
Immunochromatographic Strip (ICS) Rapid Diagnostics for Syphilis Interpretation of Results n n I Membrane strip based immunoassay Test procedure q q Recombinant T. pallidum antigen is coated on the test band region. Sample is placed on application pad and reacts with antigen-colloidal gold conjugate. If T. pallidum antibody is present an antigen-antibodyantigen gold complex will form (evident by line in the test area). Line should always appear in control region. Application Pad I Test Line Control Line Positive Sample I I Negative Sample I Inconclusive Absorbent Pad

Objectives n To evaluate ICS test performance using finger stick, whole blood, serum, and plasma specimens compared to RPR and TPPA n To compare ICS test performance with serum at local sites and CDC n To evaluate ICS test performance by syphilis stage of disease

Methods I n Enrollment- consenting adults age 18 or older n Finger stick done for point-of-care testing on 1 ICS test n Blood drawn for whole blood, serum, and plasma ICS testing at local sites and serum for CDC testing for 3 ICS tests n Data collection questionnaire includes clinical assessment of syphilis stage of disease, if applicable n Persons tested and treated for syphilis if needed according to established standard of care. n ICS tests are for investigational purposes only.

Methods II n Data analyzed using Epi-Info and SAS n Results reported by comparing to clinical standard of both RPR and TP-PA results n Sensitivity= positives/ RPR (+) TP-PA (+) n Specificity= negatives/RPR (-) TP-PA (-) n Syphilis case definitions

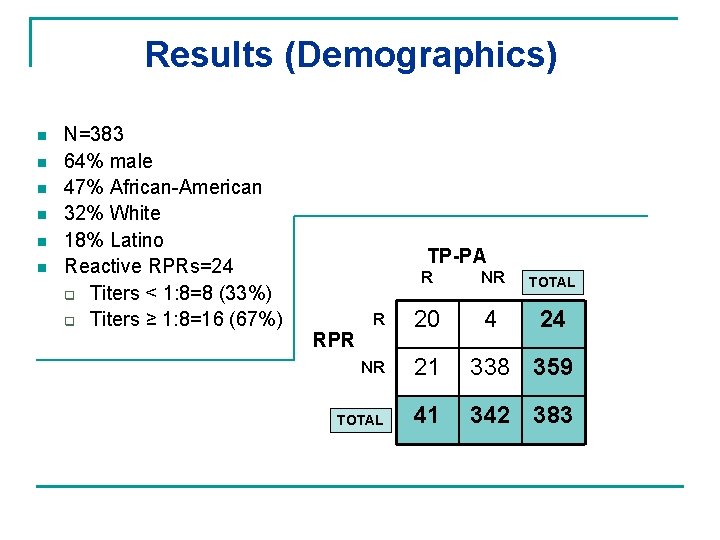
Results (Demographics) n n n N=383 64% male 47% African-American 32% White 18% Latino Reactive RPRs=24 q Titers < 1: 8=8 (33%) q Titers ≥ 1: 8=16 (67%) TP-PA R NR TOTAL R 20 4 24 NR 21 338 359 TOTAL 41 342 383 RPR

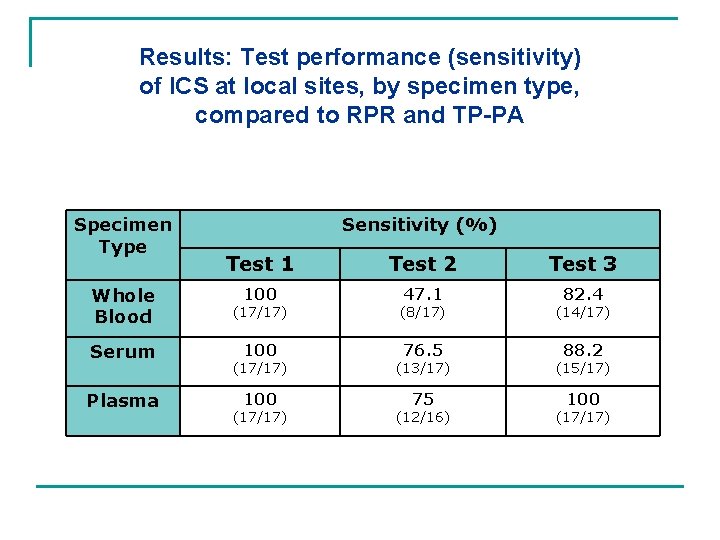
Results: Test performance (sensitivity) of ICS at local sites, by specimen type, compared to RPR and TP-PA Specimen Type Sensitivity (%) Test 1 Test 2 Test 3 100 47. 1 82. 4 Whole Blood (17/17) (8/17) (14/17) Serum 100 76. 5 88. 2 Plasma (17/17) (13/17) (15/17) 100 75 100 (17/17) (12/16) (17/17)

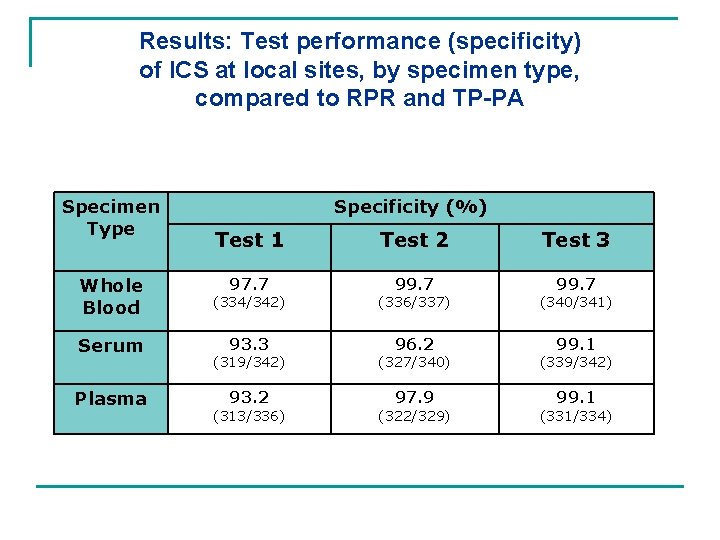
Results: Test performance (specificity) of ICS at local sites, by specimen type, compared to RPR and TP-PA Specimen Type Specificity (%) Test 1 Test 2 Test 3 97. 7 99. 7 Whole Blood (334/342) (336/337) (340/341) Serum 93. 3 96. 2 99. 1 Plasma (319/342) (327/340) (339/342) 93. 2 97. 9 99. 1 (313/336) (322/329) (331/334)

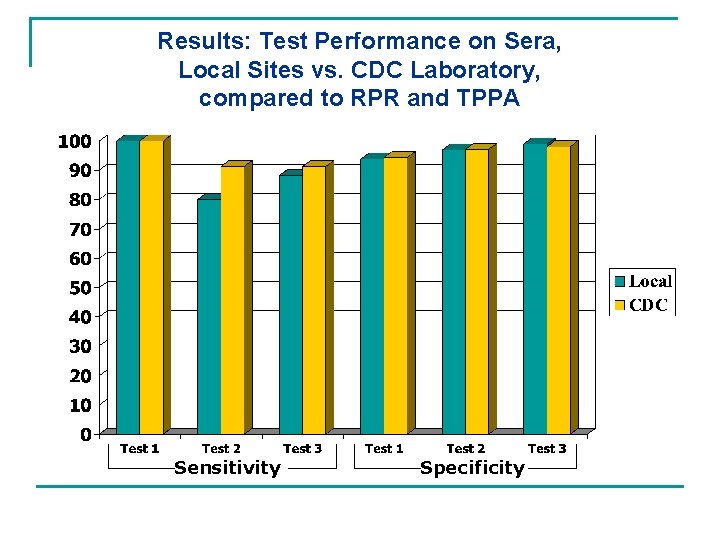
Results: Test Performance on Sera, Local Sites vs. CDC Laboratory, compared to RPR and TPPA Sensitivity Specificity

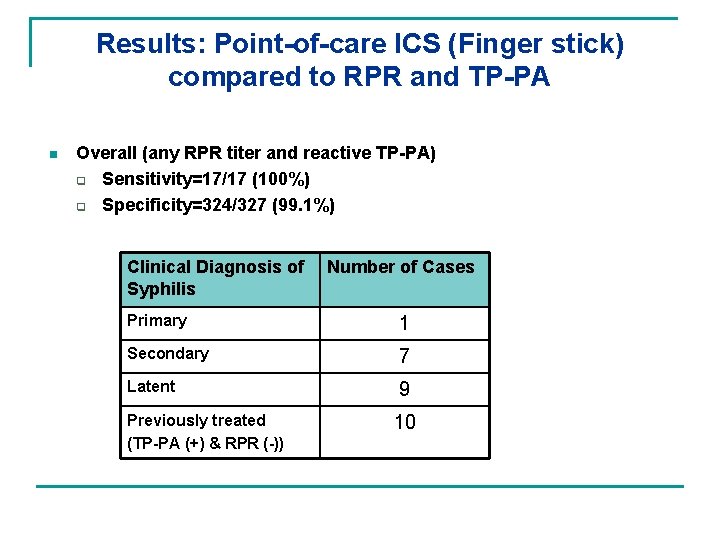
Results: Point-of-care ICS (Finger stick) compared to RPR and TP-PA n Overall (any RPR titer and reactive TP-PA) q Sensitivity=17/17 (100%) q Specificity=324/327 (99. 1%) Clinical Diagnosis of Syphilis Number of Cases Primary 1 Secondary 7 Latent 9 Previously treated (TP-PA (+) & RPR (-)) 10

Conclusions n There is a broad range of sensitivities and specificities for the 3 ICS tests in whole blood, serum, and plasma. n Test performance for sera locally is very comparable to test performance at CDC. n The ICS point-of-care (finger stick) data show sensitivities of 100% and specificities of over 99%. n ICS tests are performing well to date with various stages of disease.

Limitations n Treponemal tests are unable to differentiate between active and previously treated syphilis. n Interval analysis only; more data needed.

Summary & Future Steps n Some rapid diagnostic tests show promising performance as screening tests for syphilis in the United States. n These ICS tests require few supplies and can potentially be used in both traditional clinical settings as well as non-traditional settings. n Data collection is ongoing.

Acknowledgments n n n n n S Zackery C Ciesielski M Zajackowski M Santana C Langley L Bernard V Pope M Fears R Johnson n n n n L Markowitz J Braxton J Chapin J Lewis S Berman J Douglas B Litchfield CDC DASTLR Local Public Health Departments