Randomized Phase III Trial of Avatrombopag a Novel

Randomized Phase III Trial of Avatrombopag, a Novel Oral Thrombopoietin Receptor Agonist, in Previously Treated Chronic ITP Integrating New Hematology Findings Into Practice: Independent Conference Coverage of ASH 2017, * December 9 -12, Atlanta, Georgia *CCO is an independent medical education company that provides state-of-the-art medical information to healthcare professionals through conference coverage and other educational programs. This activity is supported by educational grants from Abb. Vie; Astra. Zeneca; Celgene Corporation; Genentech; Janssen Biotech, Inc administered by Janssen Scientific Affairs, LLC; Jazz Pharmaceuticals; Novartis Pharmaceuticals Corporation; Pharmacyclics Inc; Seattle Genetics; and Takeda Oncology.

Phase III Avatrombopag: Background § Conventional first-line therapies for ITP reduce clearance of autoantibody-coated platelets[1] – Pts failing splenectomy or second-line agents (eg, rituximab) often treated with TPO-RAs, which directly induce platelet production § Avatrombopag: small-molecule TPO-RA shown to significantly increase platelet response vs placebo by Day 28 in phase II study; generally well tolerated[2 -4] § Current phase III trial evaluated efficacy and safety of avatrombopag vs placebo in pts with refractory chronic ITP[5] References in slidenotes Slide credit: clinicaloptions. com
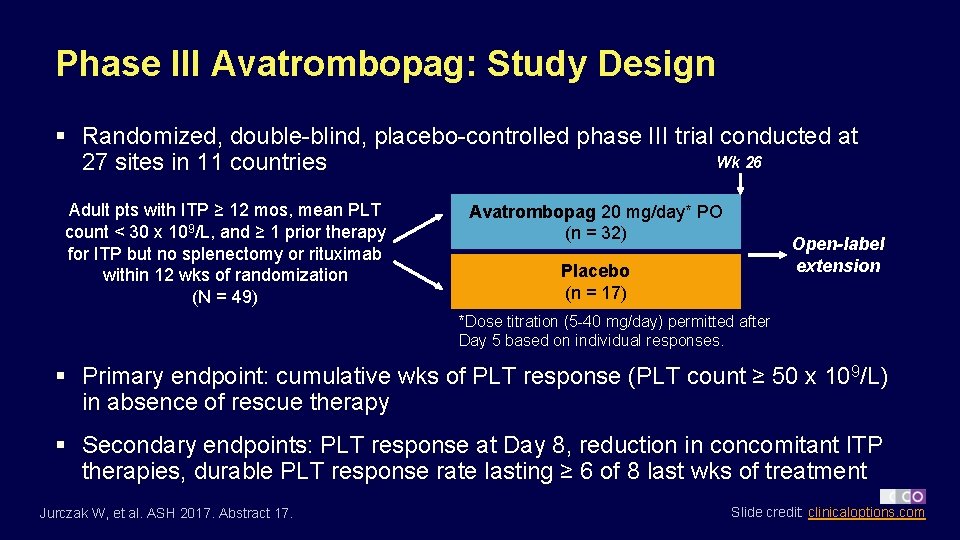
Phase III Avatrombopag: Study Design § Randomized, double-blind, placebo-controlled phase III trial conducted at Wk 26 27 sites in 11 countries Adult pts with ITP ≥ 12 mos, mean PLT count < 30 x 109/L, and ≥ 1 prior therapy for ITP but no splenectomy or rituximab within 12 wks of randomization (N = 49) Avatrombopag 20 mg/day* PO (n = 32) Open-label extension Placebo (n = 17) *Dose titration (5 -40 mg/day) permitted after Day 5 based on individual responses. § Primary endpoint: cumulative wks of PLT response (PLT count ≥ 50 x 109/L) in absence of rescue therapy § Secondary endpoints: PLT response at Day 8, reduction in concomitant ITP therapies, durable PLT response rate lasting ≥ 6 of 8 last wks of treatment Jurczak W, et al. ASH 2017. Abstract 17. Slide credit: clinicaloptions. com
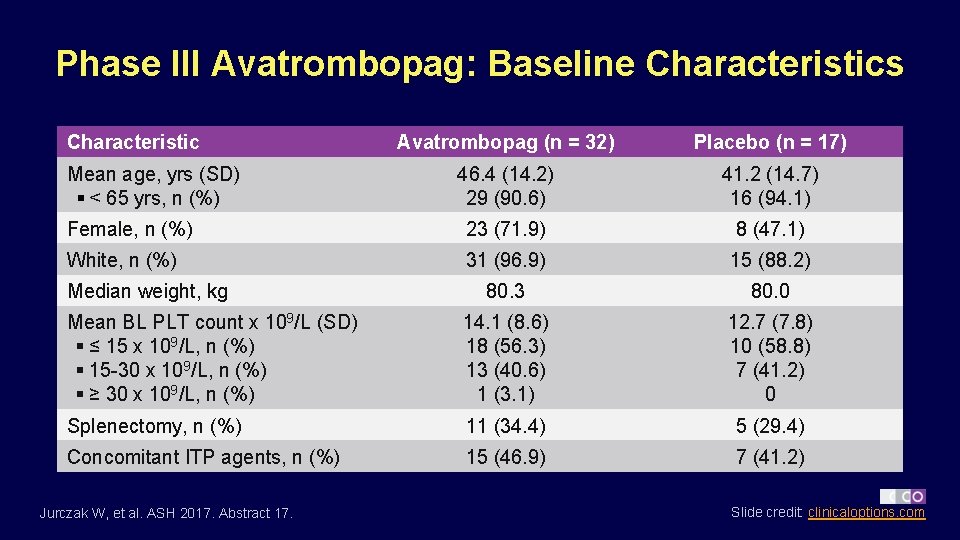
Phase III Avatrombopag: Baseline Characteristics Characteristic Avatrombopag (n = 32) Placebo (n = 17) 46. 4 (14. 2) 29 (90. 6) 41. 2 (14. 7) 16 (94. 1) Female, n (%) 23 (71. 9) 8 (47. 1) White, n (%) 31 (96. 9) 15 (88. 2) 80. 3 80. 0 Mean BL PLT count x 109/L (SD) § ≤ 15 x 109/L, n (%) § 15 -30 x 109/L, n (%) § ≥ 30 x 109/L, n (%) 14. 1 (8. 6) 18 (56. 3) 13 (40. 6) 1 (3. 1) 12. 7 (7. 8) 10 (58. 8) 7 (41. 2) 0 Splenectomy, n (%) 11 (34. 4) 5 (29. 4) Concomitant ITP agents, n (%) 15 (46. 9) 7 (41. 2) Mean age, yrs (SD) § < 65 yrs, n (%) Median weight, kg Jurczak W, et al. ASH 2017. Abstract 17. Slide credit: clinicaloptions. com
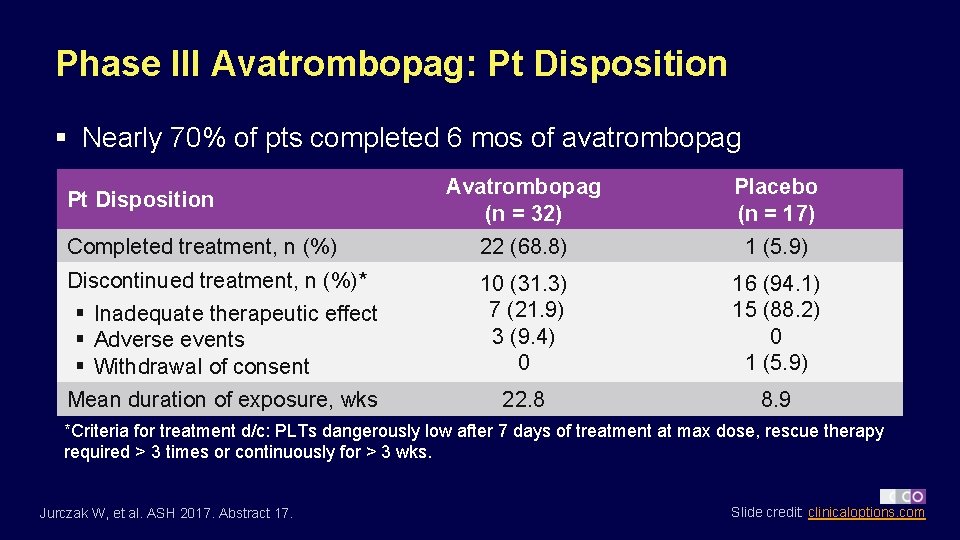
Phase III Avatrombopag: Pt Disposition § Nearly 70% of pts completed 6 mos of avatrombopag Avatrombopag (n = 32) Placebo (n = 17) Completed treatment, n (%) 22 (68. 8) 1 (5. 9) Discontinued treatment, n (%)* § Inadequate therapeutic effect § Adverse events § Withdrawal of consent 10 (31. 3) 7 (21. 9) 3 (9. 4) 0 16 (94. 1) 15 (88. 2) 0 1 (5. 9) Mean duration of exposure, wks 22. 8 8. 9 Pt Disposition *Criteria for treatment d/c: PLTs dangerously low after 7 days of treatment at max dose, rescue therapy required > 3 times or continuously for > 3 wks. Jurczak W, et al. ASH 2017. Abstract 17. Slide credit: clinicaloptions. com
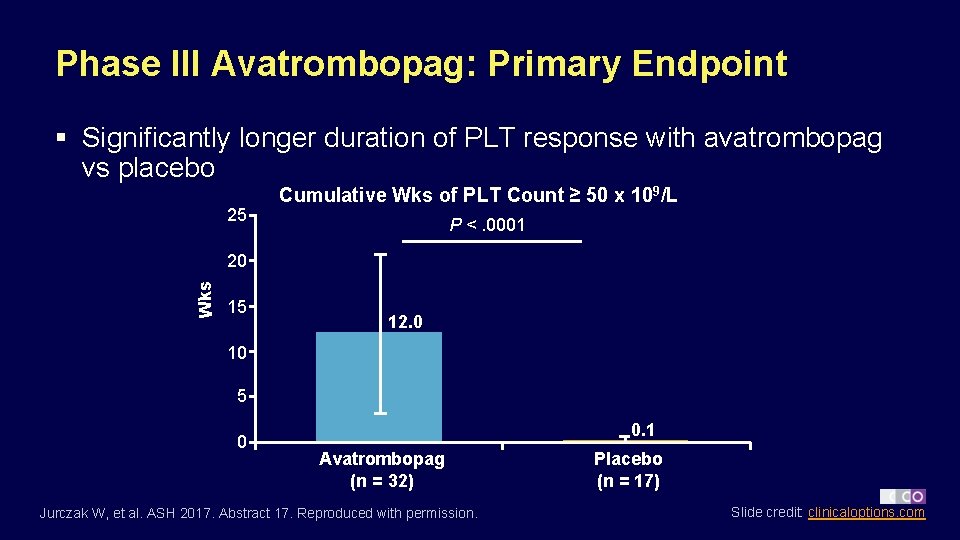
Phase III Avatrombopag: Primary Endpoint § Significantly longer duration of PLT response with avatrombopag vs placebo 25 Cumulative Wks of PLT Count ≥ 50 x 109/L P <. 0001 Wks 20 15 12. 0 10 5 0 0. 1 Avatrombopag (n = 32) Jurczak W, et al. ASH 2017. Abstract 17. Reproduced with permission. Placebo (n = 17) Slide credit: clinicaloptions. com
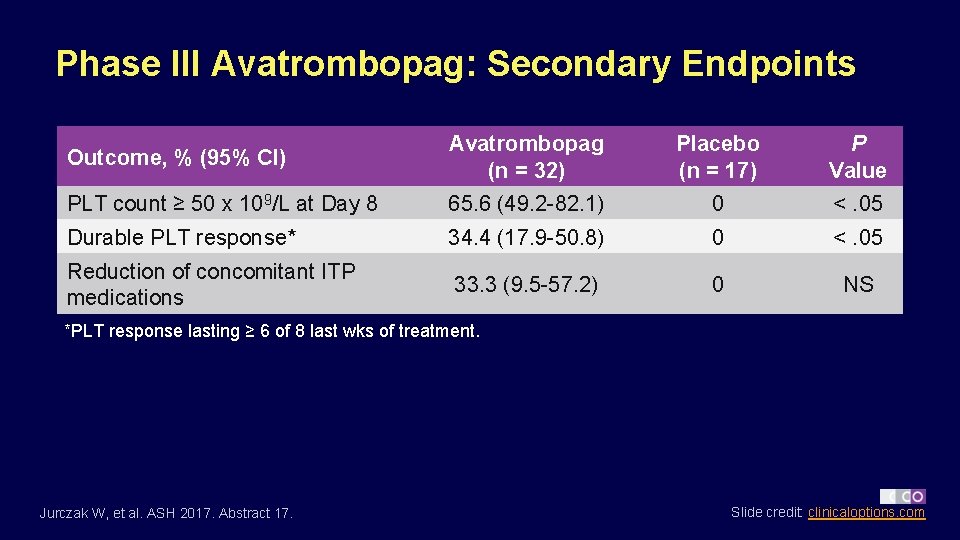
Phase III Avatrombopag: Secondary Endpoints Outcome, % (95% CI) Avatrombopag (n = 32) Placebo (n = 17) P Value PLT count ≥ 50 x 109/L at Day 8 65. 6 (49. 2 -82. 1) 0 <. 05 Durable PLT response* 34. 4 (17. 9 -50. 8) 0 <. 05 Reduction of concomitant ITP medications 33. 3 (9. 5 -57. 2) 0 NS *PLT response lasting ≥ 6 of 8 last wks of treatment. Jurczak W, et al. ASH 2017. Abstract 17. Slide credit: clinicaloptions. com
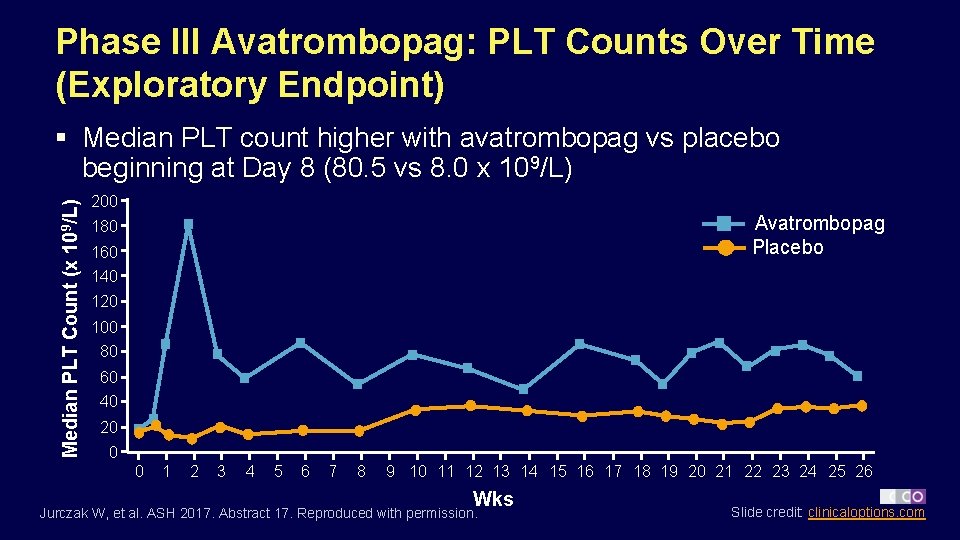
Phase III Avatrombopag: PLT Counts Over Time (Exploratory Endpoint) Median PLT Count (x 109/L) § Median PLT count higher with avatrombopag vs placebo beginning at Day 8 (80. 5 vs 8. 0 x 109/L) 200 Avatrombopag Placebo 180 160 140 120 100 80 60 40 20 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Wks Jurczak W, et al. ASH 2017. Abstract 17. Reproduced with permission. Slide credit: clinicaloptions. com
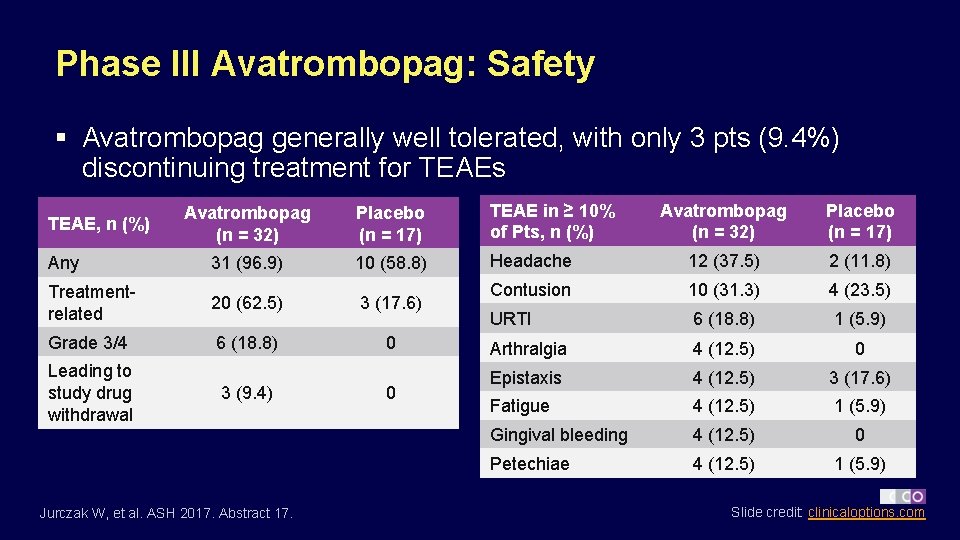
Phase III Avatrombopag: Safety § Avatrombopag generally well tolerated, with only 3 pts (9. 4%) discontinuing treatment for TEAEs Avatrombopag (n = 32) Placebo (n = 17) TEAE in ≥ 10% of Pts, n (%) Any 31 (96. 9) 10 (58. 8) Treatmentrelated 20 (62. 5) 3 (17. 6) Grade 3/4 6 (18. 8) 0 TEAE, n (%) Leading to study drug withdrawal 3 (9. 4) Jurczak W, et al. ASH 2017. Abstract 17. 0 Avatrombopag (n = 32) Placebo (n = 17) Headache 12 (37. 5) 2 (11. 8) Contusion 10 (31. 3) 4 (23. 5) URTI 6 (18. 8) 1 (5. 9) Arthralgia 4 (12. 5) 0 Epistaxis 4 (12. 5) 3 (17. 6) Fatigue 4 (12. 5) 1 (5. 9) Gingival bleeding 4 (12. 5) 0 Petechiae 4 (12. 5) 1 (5. 9) Slide credit: clinicaloptions. com
Phase III Avatrombopag: Conclusions § In pts with previously treated chronic ITP, avatrombopag superior to placebo for cumulative duration of PLT response ≥ 50 x 109/L without rescue therapy – 12. 0 vs 0. 1 wks, respectively (P <. 0001) § PLT response at Day 8 and during last 8 wks of treatment also improved with avatrombopag vs placebo § Avatrombopag well tolerated with few (9. 4%) d/c for TEAEs – Headache, contusion, upper respiratory tract infection among most commonly reported TEAES § Study investigators conclude that avatrombopag may provide a new TPO-RA treatment option for adults with refractory chronic ITP Jurczak W, et al. ASH 2017. Abstract 17. Slide credit: clinicaloptions. com

Go Online for More CCO Coverage of ASH 2017! Short slideset summaries of all the key data Additional CME-certified analyses with expert faculty commentary on all the key studies in: § § § Leukemias Lymphomas/CLL Myeloma Nonmalignant hematology Hot topics in hematology clinicaloptions. com/oncology
- Slides: 11




















