Quiz You have 10 minutes Write your name








- Slides: 22

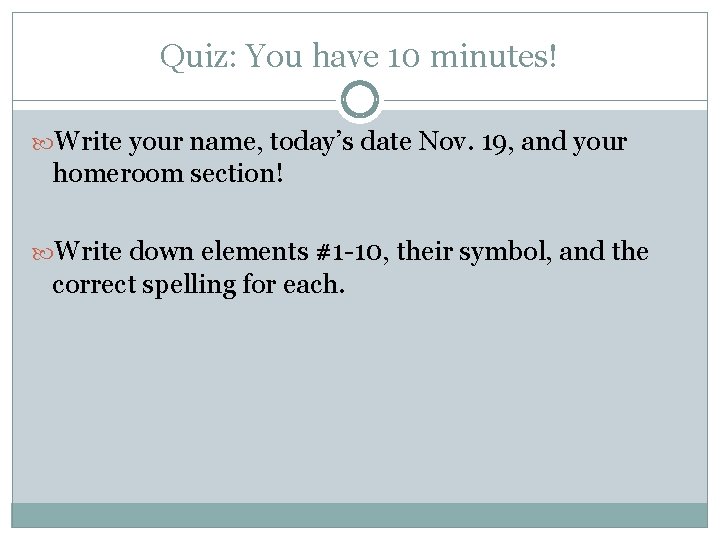
Quiz: You have 10 minutes! Write your name, today’s date Nov. 19, and your homeroom section! Write down elements #1 -10, their symbol, and the correct spelling for each.

Do Now: November 19 1. What are the group(s) of elements that are always brittle and nonmalleable? 1. What are the group(s) of elements that are shiny? 1. Compounds can be broken down only be _____ changes. 1. Is Na. Cl an element or compound? 1. Is K an element or compound?

Announcements Tonight for homework: Mixtures Packet Elements #11 -20 test Friday!!! Project due Tuesday, Dec. 3

Today’s Objectives SWBAT define mixture SWBAT identify examples of a mixtures and compounds. SWBAT describe how the characteristics of a compound are different than the characteristics of their component parts. SWBAT compare and contrast elements, compounds, and mixtures.

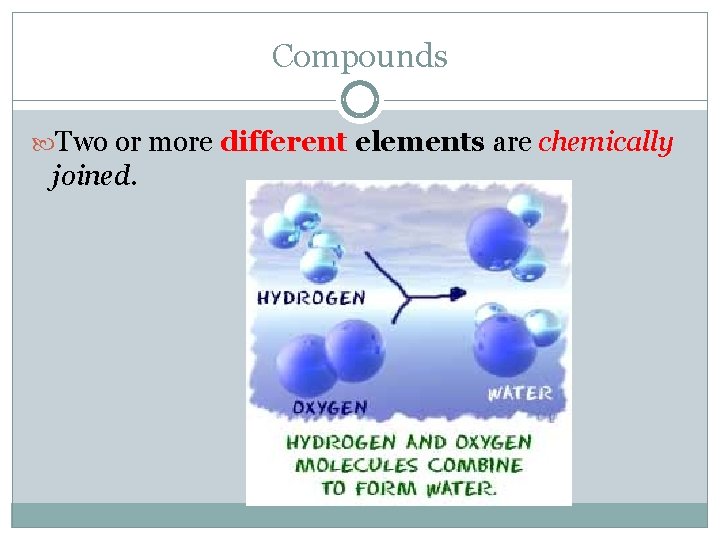
Compounds Two or more different elements are chemically joined.

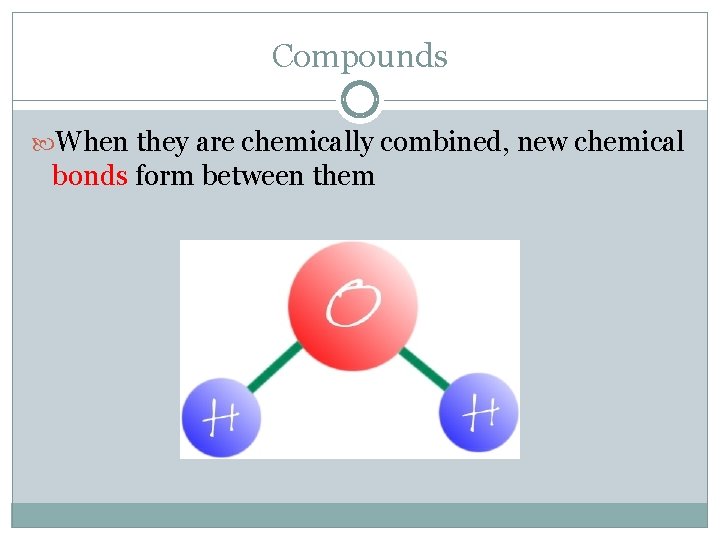
Compounds When they are chemically combined, new chemical bonds form between them

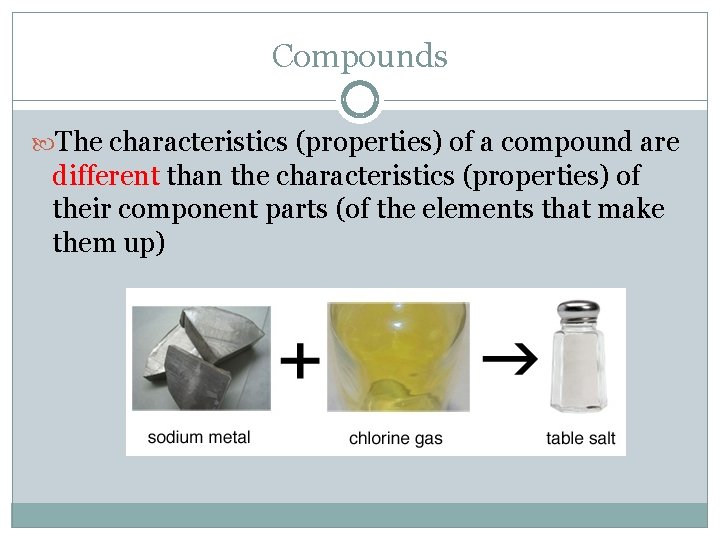
Compounds The characteristics (properties) of a compound are different than the characteristics (properties) of their component parts (of the elements that make them up)

Compounds A compound is a completely new substance or product

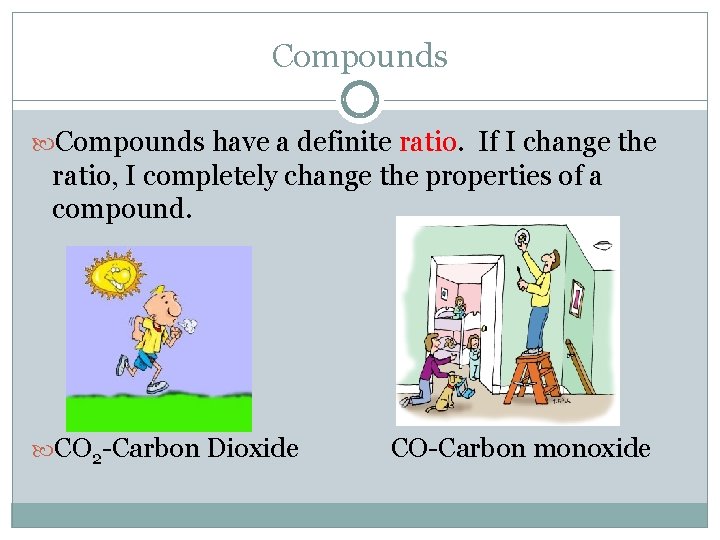
Compounds have a definite ratio. If I change the ratio, I completely change the properties of a compound. CO 2 -Carbon Dioxide CO-Carbon monoxide

Compounds Recap Two or more different elements are chemically joined When they are chemically combined, new chemical bonds form between them The characteristics (properties) of a compound are different than the characteristics (properties) of their component parts (of the elements that make them up). A compound is a completely new substance or product Compounds have a definite ratio. If I change the ratio, I completely change the properties of a compound.

Examples of Compound Salt (Na. Cl), water (H 2 O), glucose (C 6 H 12 O 6) Silver and sulfur combining to form silver sulfide


Brain. POP: mixtures

Mixtures Two or more substances are physically combined The substances in a mixture retain (keep) the properties that they originally had Physical properties can change, but there is no new substance that is formed No new chemical bonds are formed Can be easily separated into distinct parts

Examples of Mixtures Salt water Trail mix Granite Sand Soil Concrete Salad dressing

Two Types of Mixtures Heterogeneous Mixture A mixture that does not appear the same throughout Hetero=different Examples: milk and cereal Chex mix Pizza Homogenous Mixture A mixture that appears the same (uniform) throughout Homo=same Examples: Salt water coffee blood

Compound or Mixture? Food coloring in water

Compound or Mixture? Peanut butter and jelly sandwich

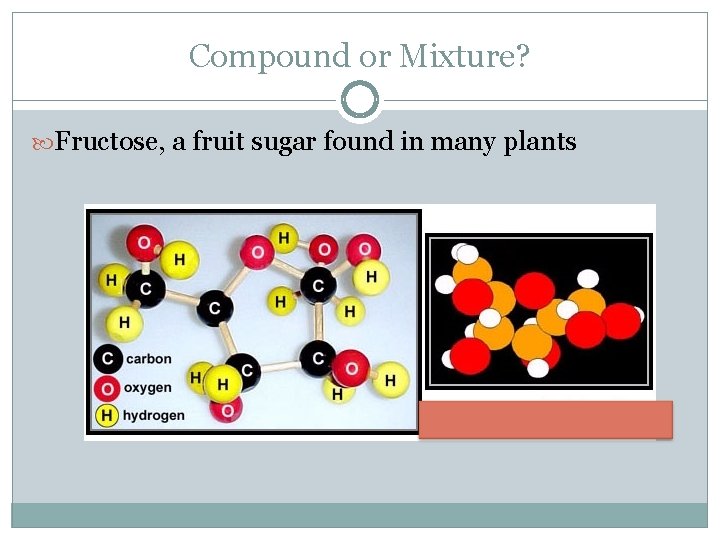
Compound or Mixture? Fructose, a fruit sugar found in many plants

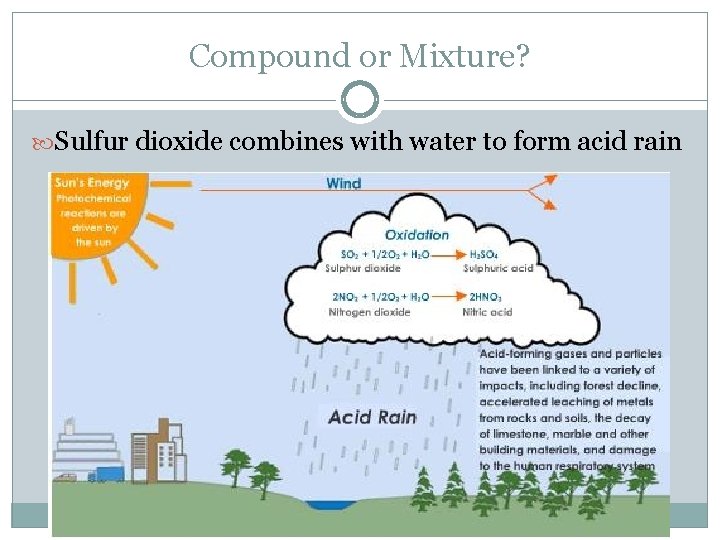
Compound or Mixture? Sulfur dioxide combines with water to form acid rain

Compound or Mixture? Oil and water

Exit Ticket: Write the answers ONLY! 1. How is a compound different from a mixture? A. mixtures are created through physical changes; compounds are created through chemical reactions B. compounds are created through physical changes; mixtures are created through chemical changes C. mixtures, on average, are heavier than compounds D. compounds, on average, are heavier than mixtures 2. What is always true of a mixture? A. it is always thicker than the two chemicals that go into it B. it retains the properties of the substances that make it up C. it can never be separated into its original substances D. it is produced through chemical reactions
 Why is a minute 60 seconds and not 100
Why is a minute 60 seconds and not 100 How old is she
How old is she In three minutes write three things
In three minutes write three things What language
What language You have 30 minutes to do this task
You have 30 minutes to do this task You have 20 minutes to do this task
You have 20 minutes to do this task You have 20 minutes to do this task
You have 20 minutes to do this task Only 10 minutes left
Only 10 minutes left You will have 40 minutes to complete the test.
You will have 40 minutes to complete the test. Be reconciled to your brother
Be reconciled to your brother I have not rejected you
I have not rejected you Conditional perfect continuous tense
Conditional perfect continuous tense Thank you for your attention do you have any questions
Thank you for your attention do you have any questions You put your left foot in
You put your left foot in You say you love rain
You say you love rain Name three lines
Name three lines Act essay prompts
Act essay prompts Hamlets moms name
Hamlets moms name Change the sentences to the negative form
Change the sentences to the negative form Daniel aleshire
Daniel aleshire Please write your name
Please write your name Which shape has 6 faces and 12 edges
Which shape has 6 faces and 12 edges Do meeting minutes have to be approved
Do meeting minutes have to be approved