Questions of the Day The reactions in our




- Slides: 14

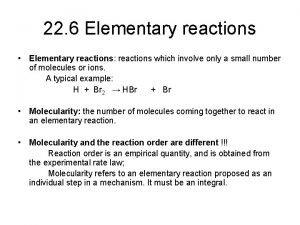
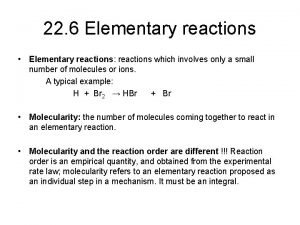
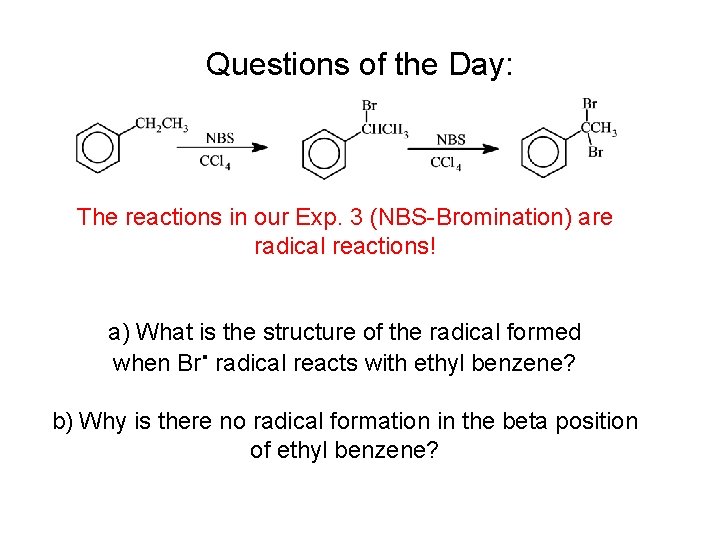
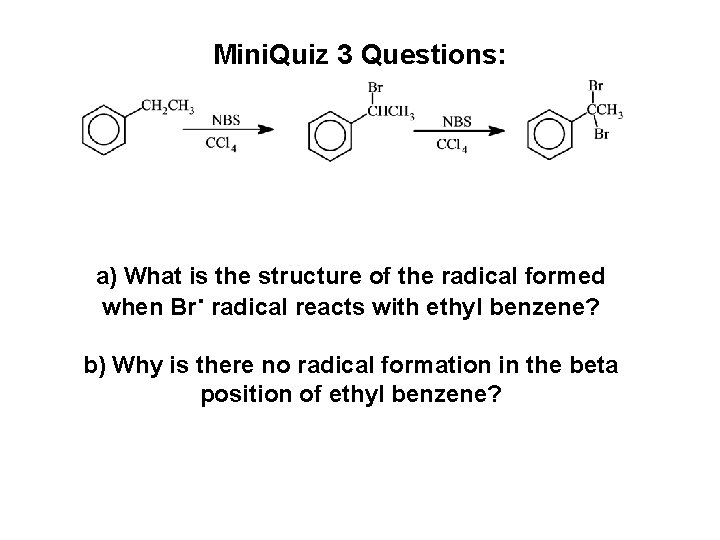
Questions of the Day: The reactions in our Exp. 3 (NBS-Bromination) are radical reactions! a) What is the structure of the radical formed when Br. radical reacts with ethyl benzene? b) Why is there no radical formation in the beta position of ethyl benzene?

Today: • Exp. 3: NBS Bromination: NMR • Introduction to Diels-Alder (Exp. 4) • Mini. Quiz

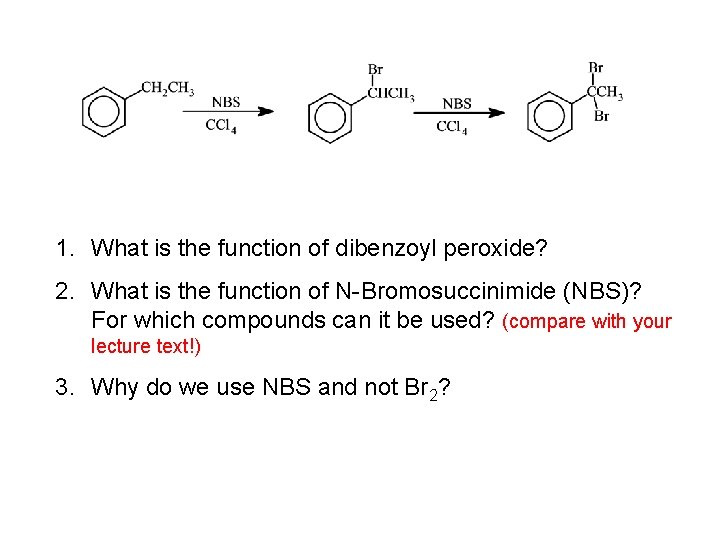
1. What is the function of dibenzoyl peroxide? 2. What is the function of N-Bromosuccinimide (NBS)? For which compounds can it be used? (compare with your lecture text!) 3. Why do we use NBS and not Br 2?

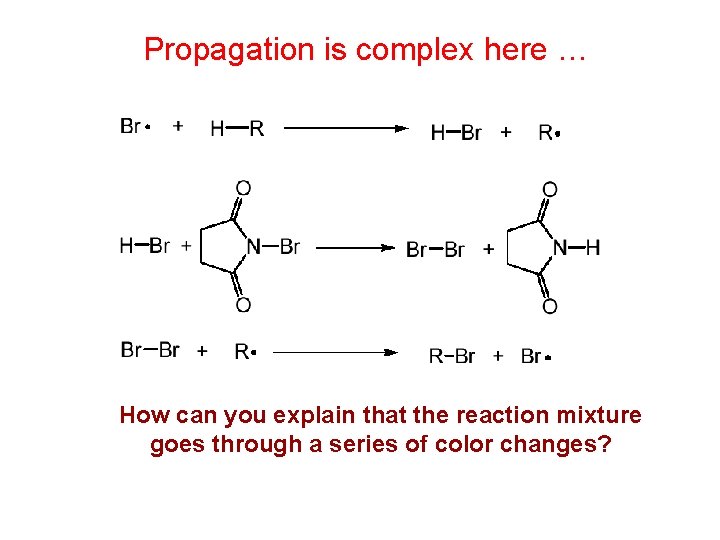
Propagation is complex here … How can you explain that the reaction mixture goes through a series of color changes?

Synthesis of Bromoethylbenzene (Manual Exp. III) 1. Why is CCl 4 a good solvent for our reaction mixture? 2. What are criteria for a "good solvent" in general? 3. What is the purpose of adding cyclododecane?

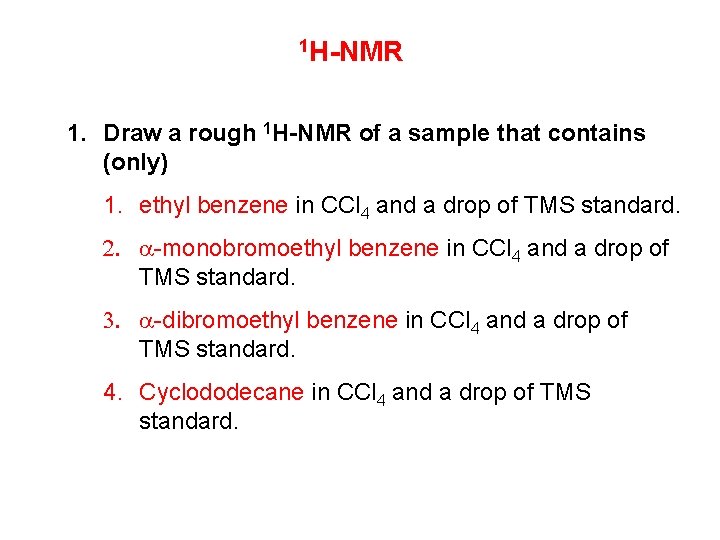
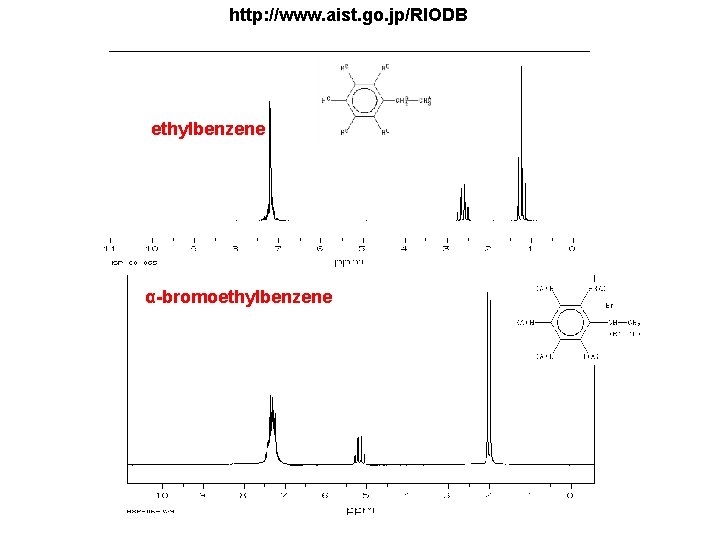
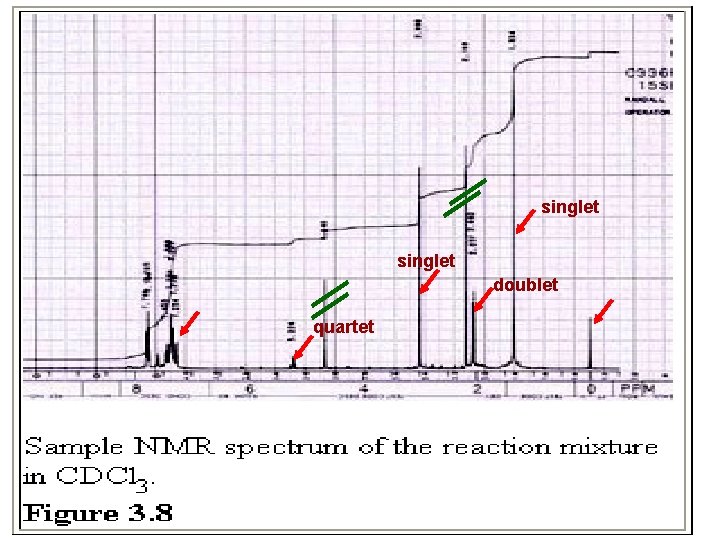
1 H-NMR 1. Draw a rough 1 H-NMR of a sample that contains (only) 1. ethyl benzene in CCl 4 and a drop of TMS standard. 2. a-monobromoethyl benzene in CCl 4 and a drop of TMS standard. 3. a-dibromoethyl benzene in CCl 4 and a drop of TMS standard. 4. Cyclododecane in CCl 4 and a drop of TMS standard.

http: //www. aist. go. jp/RIODB ethylbenzene α-bromoethylbenzene

singlet doublet quartet

1 H-NMR Why is cyclododecane a good standard in our experiment?

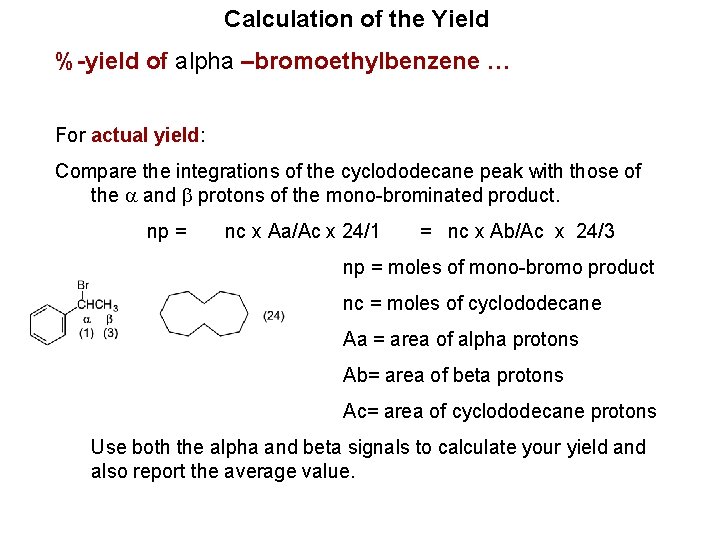
Calculation of the Yield %-yield of alpha –bromoethylbenzene … For actual yield: Compare the integrations of the cyclododecane peak with those of the a and b protons of the mono-brominated product. np = nc x Aa/Ac x 24/1 = nc x Ab/Ac x 24/3 np = moles of mono-bromo product nc = moles of cyclododecane Aa = area of alpha protons Ab= area of beta protons Ac= area of cyclododecane protons Use both the alpha and beta signals to calculate your yield and also report the average value.

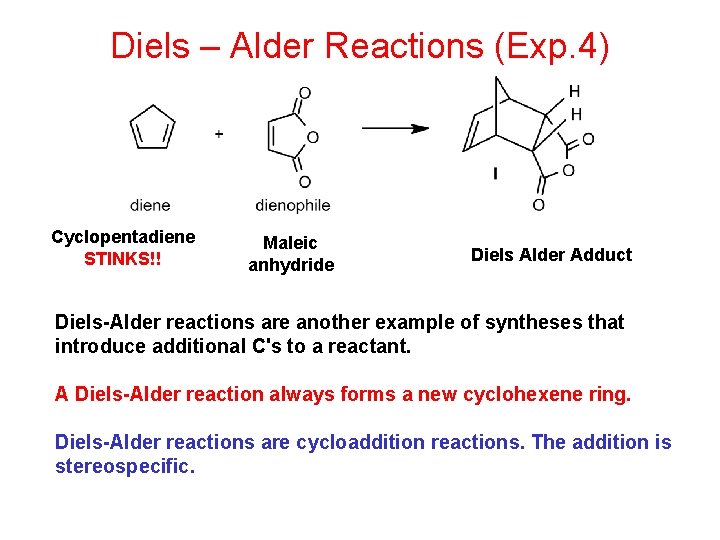
Diels – Alder Reactions (Exp. 4) Cyclopentadiene STINKS!! Maleic anhydride Diels Alder Adduct Diels-Alder reactions are another example of syntheses that introduce additional C's to a reactant. A Diels-Alder reaction always forms a new cyclohexene ring. Diels-Alder reactions are cycloaddition reactions. The addition is stereospecific.

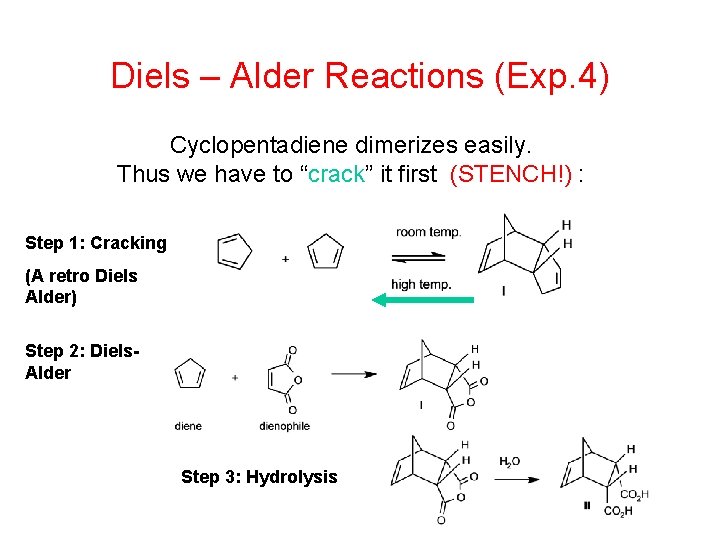
Diels – Alder Reactions (Exp. 4) Cyclopentadiene dimerizes easily. Thus we have to “crack” it first (STENCH!) : Step 1: Cracking (A retro Diels Alder) Step 2: Diels. Alder Step 3: Hydrolysis

Next time: • Diels-Alder (cont. ) • Esterification (Exp. 5) • Mini. Quiz on today’s class

Mini. Quiz 3 Questions: a) What is the structure of the radical formed when Br. radical reacts with ethyl benzene? b) Why is there no radical formation in the beta position of ethyl benzene?
 Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Day 1 day 2 day 817
Day 1 day 2 day 817 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Half redox reaction
Half redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Thinking affects our language, which then affects our:
Thinking affects our language, which then affects our: Our census our future
Our census our future Christ be our light bernadette farrell
Christ be our light bernadette farrell Marcus aurelius our life is what our thoughts make it
Marcus aurelius our life is what our thoughts make it We bow our hearts we bend our knees
We bow our hearts we bend our knees Our census our future
Our census our future Our life is what our thoughts make it
Our life is what our thoughts make it The poem money madness is written by
The poem money madness is written by