Quantitative of protein Methods of Quantitative of protein













- Slides: 15

Quantitative of protein

Methods of Quantitative of protein • Method 1: protein assay based on dye binding assay • Method 2: protein assay based on alkaline copper

Method 1: protein assay based on dye binding assay

• BRADFORD METHOD • Use of coomassie G-250 dye in a colorimetric reagent for the detection and quantitation of total protein. • In the acidic environment of the reagent, protein binds to the coomassie dye. • This results in a spectral shift from the reddish/brown form of the dye (absorbance maximum at 465 nm) to the blue form of the dye (absorbance maximum at 610 nm).

• The difference between the two forms of the dye is greatest at 595 nm, so that is the optimal wavelength to measure the blue color from the coomassie dye-protein complex • Development of color in coomassie dye-based (Bradford) protein assays has been associated with the presence of certain basic amino acids (primarily arginine, lysine and histidine) in the protein.

• Free amino acids, peptides and low molecular weight proteins do not produce color with coomassie dye reagents. (unbound) forms are green or red.


• Advantages: This assay is quick, and the reagent is not affected by the presence of reducing agents, like DTT • Disadvantages: Basic conditions and detergents, such as SDS, can interfere with the dye’s ability to bind to the protein

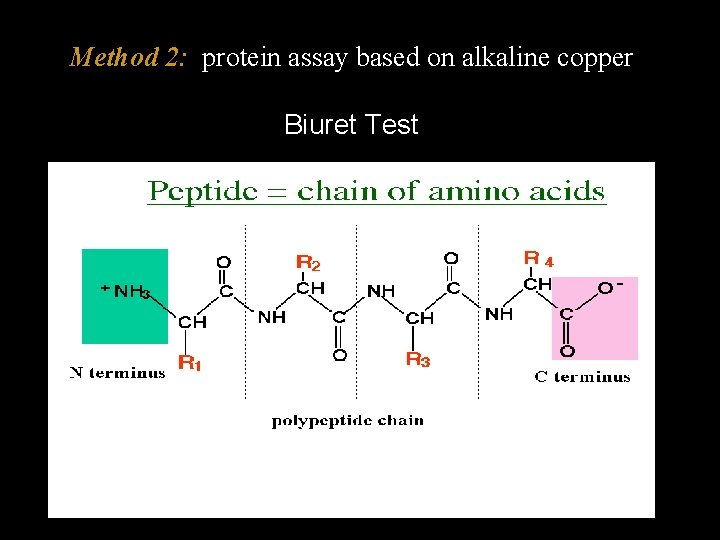
Method 2: protein assay based on alkaline copper Biuret Test

• Principle: • Under alkaline conditions substances containing two or more peptide bonds form a purple complex with copper salts in the reagent.

• Biuret Reagent contains: • Hydrated Copper sulphate – this provides the Cu (II) ions which form the chelate complex. Cu (II) ions give the reagent its characteristic blue color. • Potassium hydroxide does not participate in the reaction but provides the alkaline medium. • Potassium sodium tartrate (KNa. C 4 H 4 O 6· 4 H 2 O) stabilizes the chelate complex

• The Biuret reaction can be used to measure the concentration of proteins because peptide bonds occur with the same frequency per amino acid in the peptide. • The intensity of the color, and hence the absorption at 540 nm, is directly proportional to the protein concentration, according to the Beer-Lambert law.


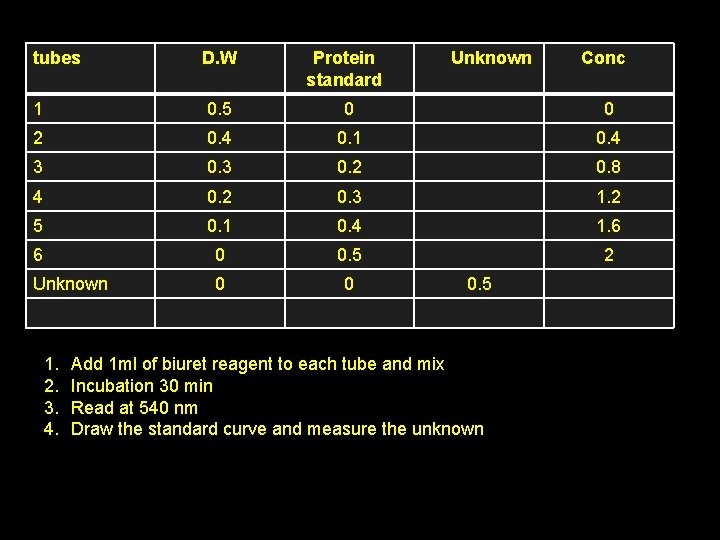
tubes D. W Protein standard 1 0. 5 0 0 2 0. 4 0. 1 0. 4 3 0. 2 0. 8 4 0. 2 0. 3 1. 2 5 0. 1 0. 4 1. 6 6 0 0. 5 2 Unknown 0 0 1. 2. 3. 4. Unknown 0. 5 Add 1 ml of biuret reagent to each tube and mix Incubation 30 min Read at 540 nm Draw the standard curve and measure the unknown Conc

Reference range • Reference range for total proteins is 66. 6 to 81. 4 g/L
 Metal coping fpd
Metal coping fpd Protein characterization techniques
Protein characterization techniques Carrier vs channel proteins
Carrier vs channel proteins Protein-protein docking
Protein-protein docking What is the sample size in qualitative research?
What is the sample size in qualitative research? Integrating qualitative and quantitative methods
Integrating qualitative and quantitative methods Protein estimation by lowry method
Protein estimation by lowry method Quantitative analysis of protein by lowry method
Quantitative analysis of protein by lowry method Categorical plan vendor rating
Categorical plan vendor rating Bond future value formula
Bond future value formula X chromosome example
X chromosome example Personality assessment methods
Personality assessment methods Hay guide chart example
Hay guide chart example Methods of acquiring knowledge in research
Methods of acquiring knowledge in research What are the four common methods of voting in ffa
What are the four common methods of voting in ffa Characteristics of good teaching methods
Characteristics of good teaching methods