Properties of Water 2 2 Water l Polar




- Slides: 9

Properties of Water 2. 2

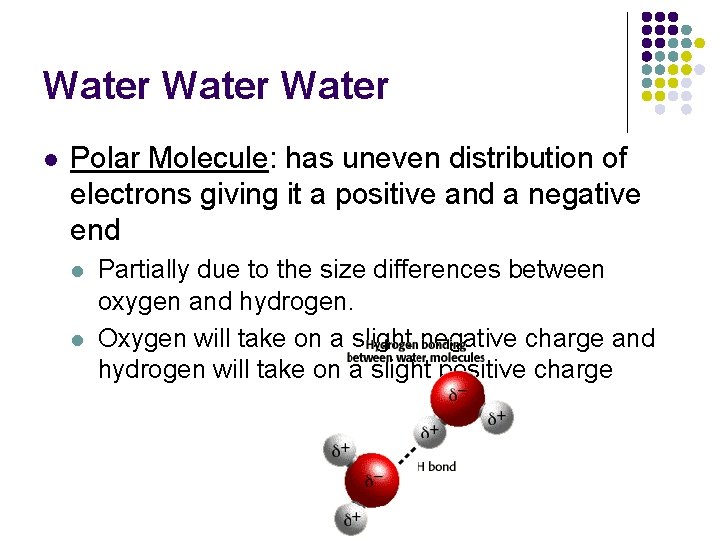
Water l Polar Molecule: has uneven distribution of electrons giving it a positive and a negative end l l Partially due to the size differences between oxygen and hydrogen. Oxygen will take on a slight negative charge and hydrogen will take on a slight positive charge

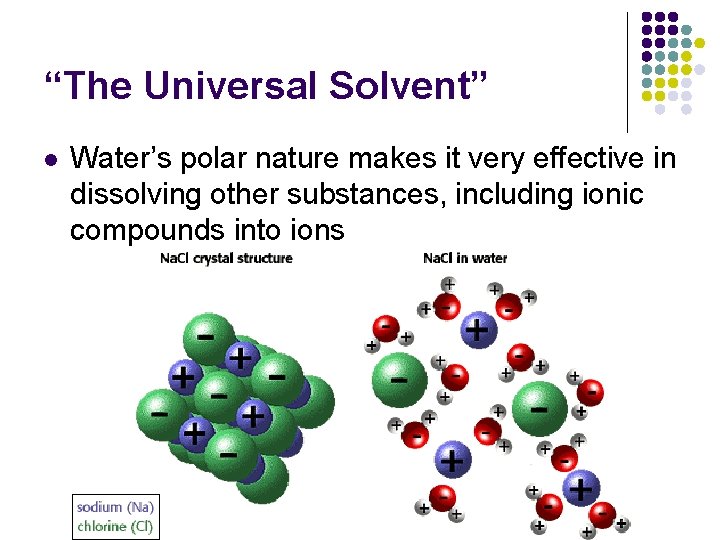
“The Universal Solvent” l Water’s polar nature makes it very effective in dissolving other substances, including ionic compounds into ions

Forms Hydrogen Bonds l l l Holds 2 H 2 O molecules together Results in Cohesion=attractive force between like particles ex: surface tension Can result in Adhesion=attraction on unlike substances l Ex: capillarity-ability for water molecules to move against gravity (think blood)

Specific Heat l l l Water needs to lose or gain high amounts of energy to break bonds and change phases How could this be helpful to organisms? Keeps cells at even temperatures during environmental changes!!!

Solutions and Suspensions l Solution: all components evenly distributed l l EX: Saltwater Salt is the SOLUTE Water is the SOLVENT Suspension: mixture of water and non dissolved particles l EX: Blood (water and cells), Fog, Muddy Water, Paint, Flour in water

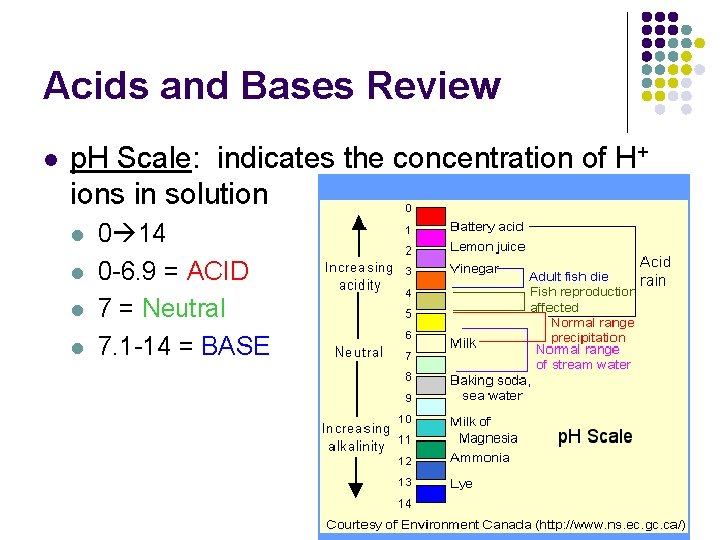
Acids and Bases Review l p. H Scale: indicates the concentration of H+ ions in solution l l 0 14 0 -6. 9 = ACID 7 = Neutral 7. 1 -14 = BASE

Acids, Bases, and Buffers l Acid: makes H+ ions in solution l l Base: makes OH- ions in solution l l p. H- below 7 The lower the number, the stronger the acid fruit juice, acetic acid (vinegar), HCl, battery acid p. H- above 7 The higher the number, the stronger the base Seawater, ammonia, bleach, oven cleaner, lye Buffers: weak acids/bases that can react with strong acids/bases to prevent major p. H changes

Diffusion l l l Net movement of molecules from hi to lo concentration http: //lessons. harveyproject. org/development/ general/diffusion/diffnomemb. ht ml http: //www. biosci. ohiou. edu/introbioslab/Bios 170/diffusion/Diffusion. html