PROPERTIES OF MATTER INTRODUCTION TO CHEMISTRY PROPERTIES OF





- Slides: 7

PROPERTIES OF MATTER INTRODUCTION TO CHEMISTRY

PROPERTIES OF MATTER • A property is a characteristic that may help to identify a substance. – We call this properties of matter. • Qualitative (observable) Properties – properties that you can observe with your senses – Sight , taste, hear, touch, smell – Describe with words • Quantitative (measurable) Properties – properties that you can measure – Associated with numbers and units – Observations made with instruments such as rulers, balances, graduated cylinders, beakers, and thermometers.

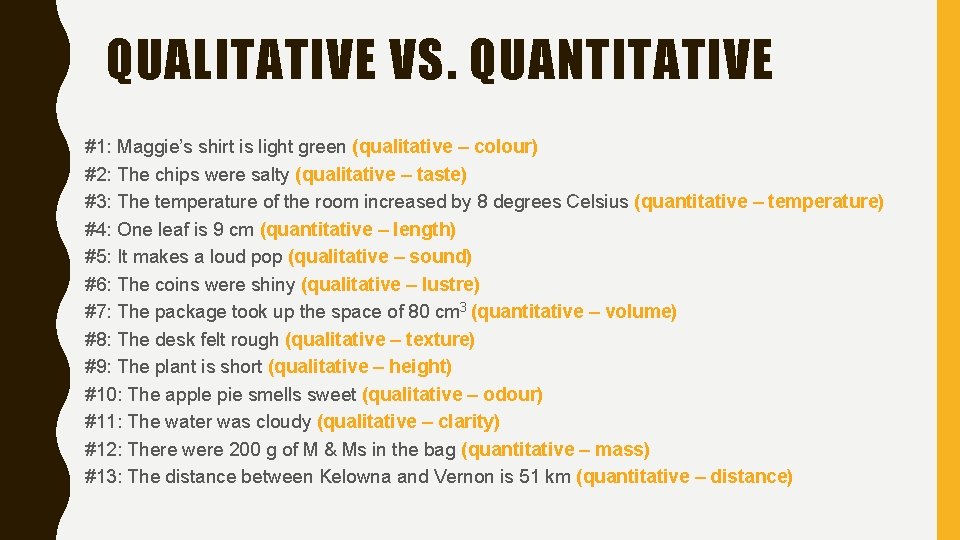
CHOOSE IF QUALITATIVE OR QUANTITATIVE #1: Maggie’s shirt is light green #2: The chips were salty #3: The temperature of the room increased by 8 degrees Celsius #4: One leaf is 9 cm #5: It makes a loud pop #6: The coins were shiny #7: The package took up the space of 80 cm 3 #8: The desk felt rough #9: The plant is short #10: The apple pie smells sweet #11: The water was cloudy #12: There were 200 g of M & Ms in the bag #13: The distance between Kelowna and Vernon is 51 km

QUALITATIVE VS. QUANTITATIVE #1: Maggie’s shirt is light green (qualitative – colour) #2: The chips were salty (qualitative – taste) #3: The temperature of the room increased by 8 degrees Celsius (quantitative – temperature) #4: One leaf is 9 cm (quantitative – length) #5: It makes a loud pop (qualitative – sound) #6: The coins were shiny (qualitative – lustre) #7: The package took up the space of 80 cm 3 (quantitative – volume) #8: The desk felt rough (qualitative – texture) #9: The plant is short (qualitative – height) #10: The apple pie smells sweet (qualitative – odour) #11: The water was cloudy (qualitative – clarity) #12: There were 200 g of M & Ms in the bag (quantitative – mass) #13: The distance between Kelowna and Vernon is 51 km (quantitative – distance)

PROPERTIES OF MATTER THAT YOU OBSERVE – QUALITATIVE PROPERTIES • Colour – What colour(s) is the substance? Try and be specific and scientific. • State – Is the matter a solid, liquid, or gas? • Malleability – Can the substance be pounded into a sheet? • Ductility – Can the substance be stretched into a wire? • Luster – Is the substance shiny? Is it dull? • Magnetism – Is the substance magnetic? • Odour – Does it have sweet smell? Burnt? • Texture – Is it smooth or rough? How does it feel? • Taste – Is sweet, salty, sour?

PROPERTIES OF MATTER THAT YOU MEASURE – QUANTITATIVE PROPERTIES • • • Mass – how much matter is in an object? Volume – how much space does an object take up? Distance – what is the object’s length? Density – How dense is the substance? Does it float in water? Melting/Freezing/Boiling Point – At what temperature will the substance change state? – Melting point of a substance is the temperature at which a solid form of a substance changes to a liquid. – Freezing point of a substance is the temperature at which a liquid form of a substance changes to a solid. – Boiling point of a substance is the temperature at which a liquid form of a substance changes to a gas. • Viscosity – How fast can a liquid pour? • Conductivity – Can the substance conduct heat or electricity? • Solubility – How much/how fast can a substance dissolve?

I SPY • Play “I Spy” with a partner using the observable properties of matter. Use the format, “I spy something that is (pick a state) and is (pick one or more qualitative properties…” For example, “I spy something that is solid, blue, and rectangular. What is it? ”
 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Grey matter nervous system
Grey matter nervous system Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Dural septa
Dural septa Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Grey matter and white matter in brain
Grey matter and white matter in brain