Progress Report Accurate line intensities for 16 O
![Sample spectrum capturing various types of CO 2 isotopologues [626]; [627]; [628]; [727] This Sample spectrum capturing various types of CO 2 isotopologues [626]; [627]; [628]; [727] This](https://slidetodoc.com/presentation_image_h2/98351d2abfdb53568a8c5c6f5efd331a/image-8.jpg)

- Slides: 13

Progress Report Accurate line intensities for 16 O 12 C 17 O (627) in the 2. 1 µm region (the OCO-2 strong band) David Jacquemart 1, 2, Keeyoon Sung 3, Linda R. Brown 3, Arlan W. Mantz 4, Mary Ann H. Smith 5 UPMC Univ Paris 06, Laboratoire de Dynamique, Interactions et Réactivité, Paris, France. CNRS, UMR 7075, Laboratoire de Dynamique, Interactions et Réactivité, Paris, France. 3 Jet Propulsion Laboratory/California Institute of Technology, Pasadena, CA 4 Dept. of Physics, Astronomy and Geophysics, Connecticut College, New London, CT 5 Science Directorate, NASA Langley Research Center, Hampton, VA 1 2 JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 1/13

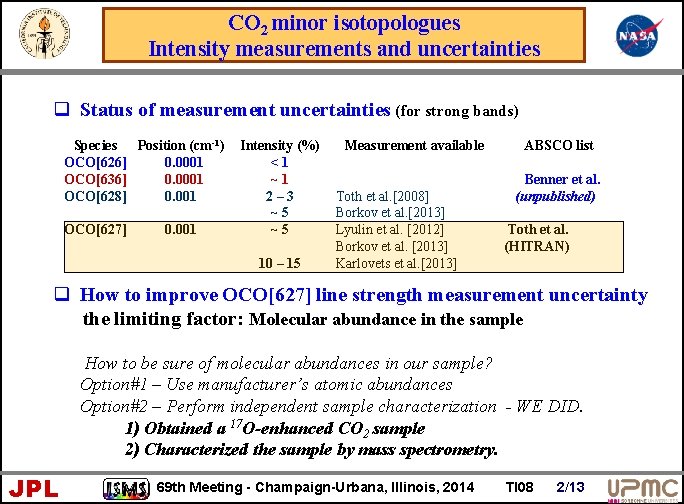
CO 2 minor isotopologues Intensity measurements and uncertainties q Status of measurement uncertainties (for strong bands) Species Position (cm-1) OCO[626] 0. 0001 OCO[636] 0. 0001 OCO[628] 0. 001 OCO[627] 0. 001 Intensity (%) <1 ~1 2– 3 ~5 ~5 10 – 15 Measurement available Toth et al. [2008] Borkov et al. [2013] Lyulin et al. [2012] Borkov et al. [2013] Karlovets et al. [2013] ABSCO list Benner et al. (unpublished) Toth et al. (HITRAN) q How to improve OCO[627] line strength measurement uncertainty the limiting factor: Molecular abundance in the sample How to be sure of molecular abundances in our sample? Option#1 – Use manufacturer’s atomic abundances Option#2 – Perform independent sample characterization - WE DID. 1) Obtained a 17 O-enhanced CO 2 sample 2) Characterized the sample by mass spectrometry. JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 2/13

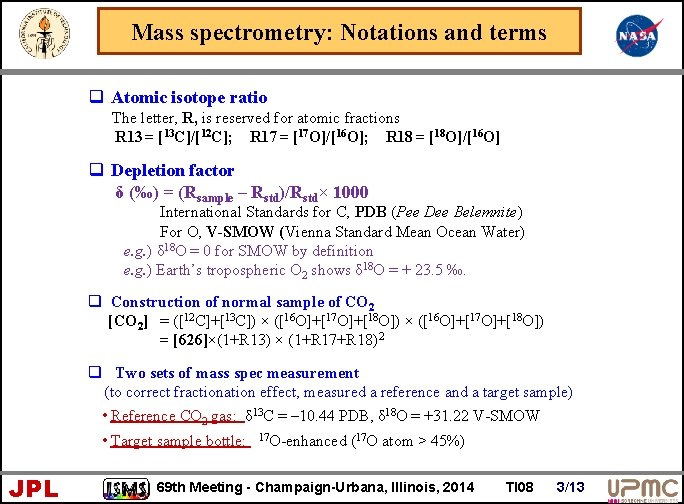
Mass spectrometry: Notations and terms q Atomic isotope ratio The letter, R, is reserved for atomic fractions R 13 = [13 C]/[12 C]; R 17 = [17 O]/[16 O]; R 18 = [18 O]/[16 O] q Depletion factor δ (‰) = (Rsample – Rstd)/Rstd× 1000 International Standards for C, PDB (Pee Dee Belemnite) For O, V-SMOW (Vienna Standard Mean Ocean Water) e. g. ) δ 18 O = 0 for SMOW by definition e. g. ) Earth’s tropospheric O 2 shows δ 18 O = + 23. 5 ‰. q Construction of normal sample of CO 2 [CO 2] = ([12 C]+[13 C]) × ([16 O]+[17 O]+[18 O]) = [626]×(1+R 13) × (1+R 17+R 18)2 q Two sets of mass spec measurement (to correct fractionation effect, measured a reference and a target sample) • Reference CO 2 gas: δ 13 C = – 10. 44 PDB, δ 18 O = +31. 22 V-SMOW • Target sample bottle: JPL 17 O-enhanced (17 O atom > 45%) 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 3/13

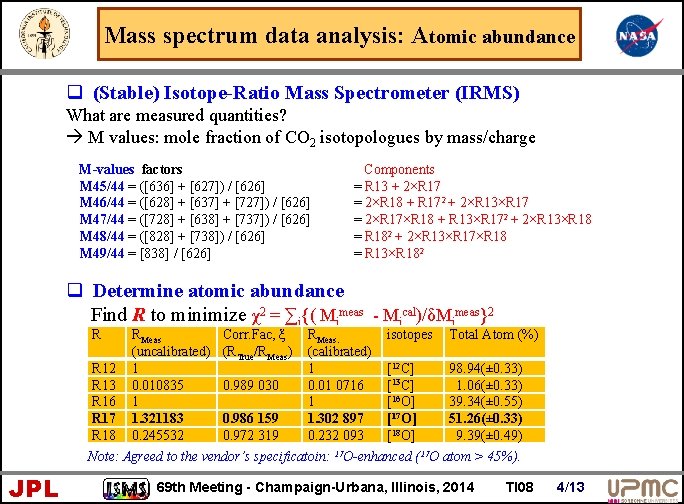
Mass spectrum data analysis: Atomic abundance q (Stable) Isotope-Ratio Mass Spectrometer (IRMS) What are measured quantities? à M values: mole fraction of CO 2 isotopologues by mass/charge M-values factors M 45/44 = ([636] + [627]) / [626] M 46/44 = ([628] + [637] + [727]) / [626] M 47/44 = ([728] + [638] + [737]) / [626] M 48/44 = ([828] + [738]) / [626] M 49/44 = [838] / [626] Components = R 13 + 2×R 17 = 2×R 18 + R 172 + 2×R 13×R 17 = 2×R 17×R 18 + R 13×R 172 + 2×R 13×R 18 = R 182 + 2×R 13×R 17×R 18 = R 13×R 182 q Determine atomic abundance Find R to minimize χ2 = ∑i{( Mimeas - Mical)/δMimeas}2 R R 12 R 13 R 16 R 17 R 18 RMeas (uncalibrated) 1 0. 010835 1 1. 321183 0. 245532 Corr. Fac, ξ (RTrue/RMeas) 0. 989 030 0. 986 159 0. 972 319 RMeas. (calibrated) 1 0. 01 0716 1 1. 302 897 0. 232 093 isotopes Total Atom (%) [12 C] [13 C] [16 O] [17 O] [18 O] 98. 94(± 0. 33) 1. 06(± 0. 33) 39. 34(± 0. 55) 51. 26(± 0. 33) 9. 39(± 0. 49) Note: Agreed to the vendor’s specificatoin: 17 O-enhanced (17 O atom > 45%). JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 4/13

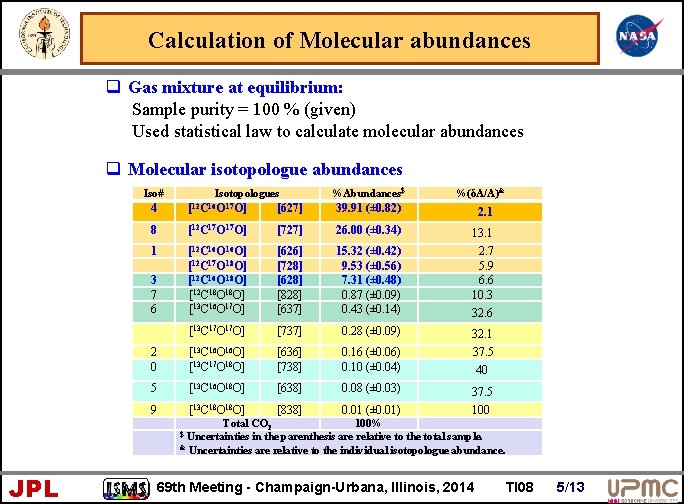
Calculation of Molecular abundances q Gas mixture at equilibrium: Sample purity = 100 % (given) Used statistical law to calculate molecular abundances q Molecular isotopologue abundances Iso# Isotopologues %Abundances$ %(δA/A)& 4 [12 C 16 O 17 O] [627] 39. 91 (± 0. 82) 2. 1 8 [12 C 17 O] [727] 26. 00 (± 0. 34) 1 [12 C 16 O] [12 C 16 O 18 O] [12 C 18 O] [13 C 16 O 17 O] [626] [728] [628] [828] [637] 15. 32 (± 0. 42) 9. 53 (± 0. 56) 7. 31 (± 0. 48) 0. 87 (± 0. 09) 0. 43 (± 0. 14) 13. 1 2. 7 5. 9 6. 6 10. 3 32. 6 [13 C 17 O] [737] 0. 28 (± 0. 09) 2 0 [13 C 16 O] [13 C 17 O 18 O] [636] [738] 0. 16 (± 0. 06) 0. 10 (± 0. 04) 5 [13 C 16 O 18 O] [638] 0. 08 (± 0. 03) 9 [13 C 18 O] [838] 0. 01 (± 0. 01) [12 C 17 O 18 O] 3 7 6 32. 1 37. 5 40 37. 5 100 Total CO 2 100% $ Uncertainties in the parenthesis are relative to the total sample. & Uncertainties are relative to the individual isotopologue abundance. JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 5/13

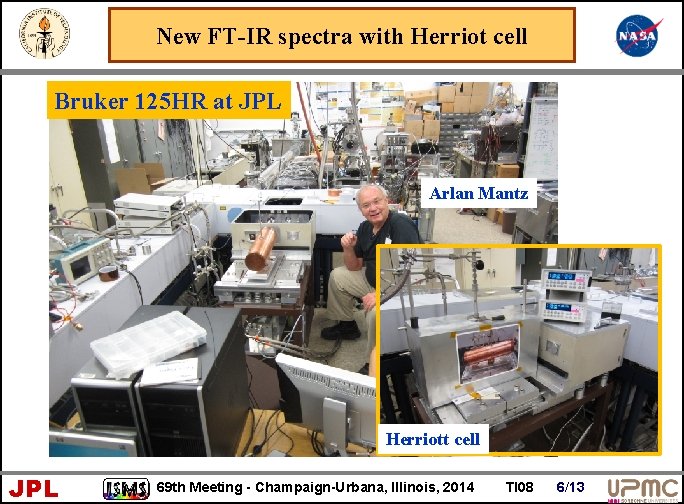
New FT-IR spectra with Herriot cell Bruker 125 HR at JPL Arlan Mantz Herriott cell JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 6/13

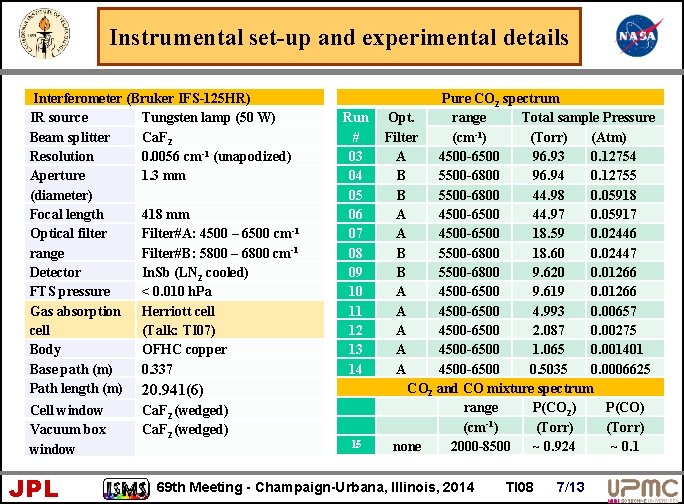
Instrumental set-up and experimental details Interferometer (Bruker IFS-125 HR) IR source Tungsten lamp (50 W) Beam splitter Ca. F 2 Resolution 0. 0056 cm-1 (unapodized) Aperture 1. 3 mm (diameter) Focal length 418 mm Optical filter Filter#A: 4500 – 6500 cm-1 range Filter#B: 5800 – 6800 cm-1 Detector In. Sb (LN 2 cooled) FTS pressure < 0. 010 h. Pa Gas absorption Herriott cell (Talk: TI 07) Body OFHC copper Base path (m) 0. 337 Path length (m) 20. 941(6) Cell window Vacuum box window JPL Ca. F 2 (wedged) Run # 03 04 05 06 07 08 09 10 11 12 13 14 15 Pure CO 2 spectrum Opt. range Total sample Pressure (cm-1) (Torr) (Atm) Filter A 4500 -6500 96. 93 0. 12754 B 5500 -6800 96. 94 0. 12755 B 5500 -6800 44. 98 0. 05918 A 4500 -6500 44. 97 0. 05917 A 4500 -6500 18. 59 0. 02446 B 5500 -6800 18. 60 0. 02447 B 5500 -6800 9. 620 0. 01266 A 4500 -6500 9. 619 0. 01266 A 4500 -6500 4. 993 0. 00657 A 4500 -6500 2. 087 0. 00275 A 4500 -6500 1. 065 0. 001401 A 4500 -6500 0. 5035 0. 0006625 CO 2 and CO mixture spectrum range P(CO 2) P(CO) -1 (cm ) (Torr) none 2000 -8500 ~ 0. 924 ~ 0. 1 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 7/13
![Sample spectrum capturing various types of CO 2 isotopologues 626 627 628 727 This Sample spectrum capturing various types of CO 2 isotopologues [626]; [627]; [628]; [727] This](https://slidetodoc.com/presentation_image_h2/98351d2abfdb53568a8c5c6f5efd331a/image-8.jpg)
Sample spectrum capturing various types of CO 2 isotopologues [626]; [627]; [628]; [727] This work: 2 µm region L = 20. 941 m P = 0. 5 – 98 Torr T = 296 K Resnl = 0. 0056 cm-1 JPL Future work Residual H 2 O HCl 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 8/13

Multispectrum fitting - Required a multispectrum fitting procedure - Employed the program by Jacquemart et al. (2002) JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 9/13

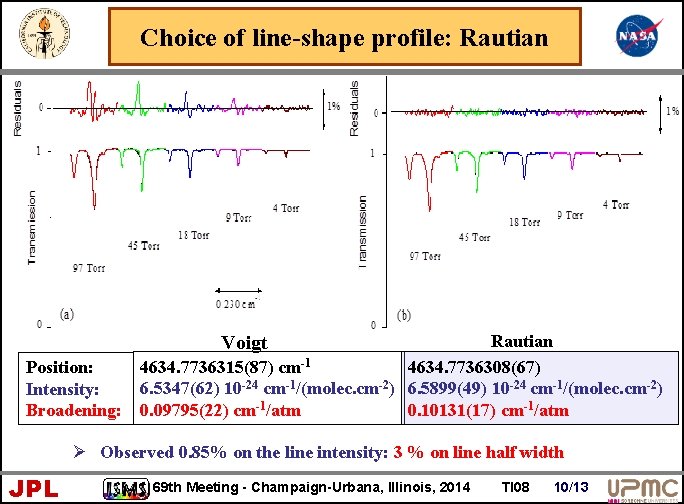
Choice of line-shape profile: Rautian 4634. 7736315(87) cm-1 4634. 7736308(67) 6. 5347(62) 10 -24 cm-1/(molec. cm-2) 6. 5899(49) 10 -24 cm-1/(molec. cm-2) 0. 09795(22) cm-1/atm 0. 10131(17) cm-1/atm Voigt Position: Intensity: Broadening: Ø Observed 0. 85% on the line intensity: 3 % on line half width JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 10/13

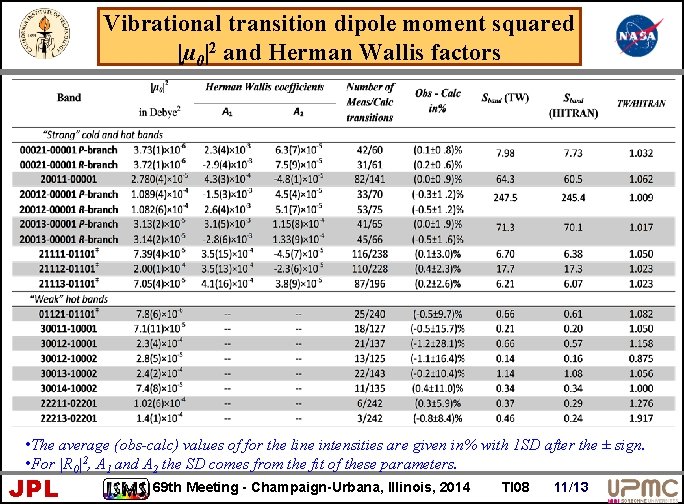
Vibrational transition dipole moment squared |µ 0|2 and Herman Wallis factors • The average (obs-calc) values of for the line intensities are given in% with 1 SD after the ± sign. • For |R 0|2, A 1 and A 2 the SD comes from the fit of these parameters. JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 11/13

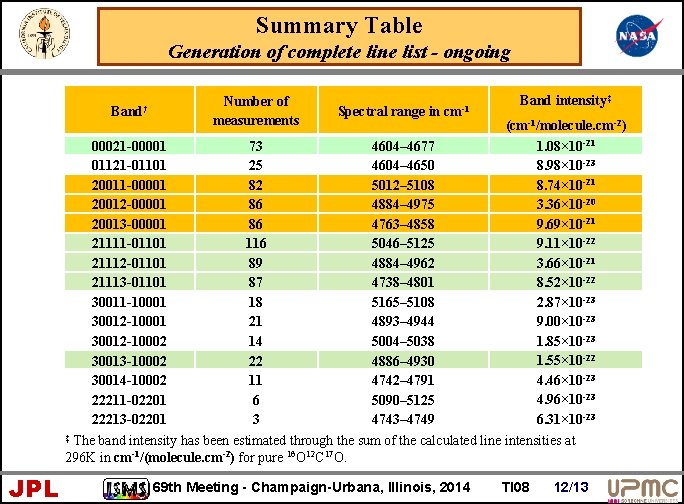
Summary Table Generation of complete line list - ongoing Band† Number of measurements Spectral range in cm-1 Band intensity‡ (cm-1/molecule. cm-2) 1. 08× 10 -21 00021 -00001 73 4604– 4677 8. 98× 10 -23 01121 -01101 25 4604– 4650 8. 74× 10 -21 20011 -00001 82 5012– 5108 3. 36× 10 -20 20012 -00001 86 4884– 4975 9. 69× 10 -21 20013 -00001 86 4763– 4858 9. 11× 10 -22 21111 -01101 116 5046– 5125 3. 66× 10 -21 21112 -01101 89 4884– 4962 8. 52× 10 -22 21113 -01101 87 4738– 4801 2. 87× 10 -23 30011 -10001 18 5165– 5108 9. 00× 10 -23 30012 -10001 21 4893– 4944 1. 85× 10 -23 30012 -10002 14 5004– 5038 1. 55× 10 -22 30013 -10002 22 4886– 4930 4. 46× 10 -23 30014 -10002 11 4742– 4791 4. 96× 10 -23 22211 -02201 6 5090– 5125 22213 -02201 3 4743– 4749 6. 31× 10 -23 ‡ The band intensity has been estimated through the sum of the calculated line intensities at 296 K in cm-1/(molecule. cm-2) for pure 16 O 12 C 17 O. JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 12/13

Thank you for your attention Acknowledgements Research described in this talk was performed at UPMC/CNRS (France), Connecticut College, NASA Langley Research Center and the Jet Propulsion Laboratory, California Institute of Technology, under contracts and cooperative agreements with the National Aeronautics and Space Administration. We also thank Chip Miller for providing the 17 O-enriched CO 2 sample, Max Coleman for the mass spec measurements of the CO 2 sample, Tim Crawford for the technical assistance on the data acquisition with a FT-IR. JPL 69 th Meeting - Champaign-Urbana, Illinois, 2014 TI 08 13/13