Prof Hanan Hagar Pharmacology Department Is the fraction





















- Slides: 32

Prof. Hanan Hagar Pharmacology Department

Is the fraction of unchanged drug that enters systemic circulation after administration and becomes available to produce an action I. V. provides 100% bioavailability. Oral usually has less than I. V.

The bioavailability of a drug product is compared to its intravenous standard formulation.

This calculation is determined when two products are compared to each other, not to an intravenous standard. E. g Tylenol (paracetamol 500 mg) compared to panadol (paracetamol 500 mg)

This is commonly calculated in the generic drug industry to determine that the generic formulation (e. g. , a tablet) is bioequivalent to the original formulation (e. g. , another tablet). Pharmaceutical industries conduct bioequivalence studies for their new product to decide on formulation for the clinical use.

Two drug products are considered to be bioequivalent when the rates and extents of bioavailability of the active ingredient in the two products are not significantly different under suitable test conditions.

What student should know Major body fluid compartments Concept of compartments. Apparent volume of distribution (vd). Plasma protein binding. Tissue binding. Redistribution

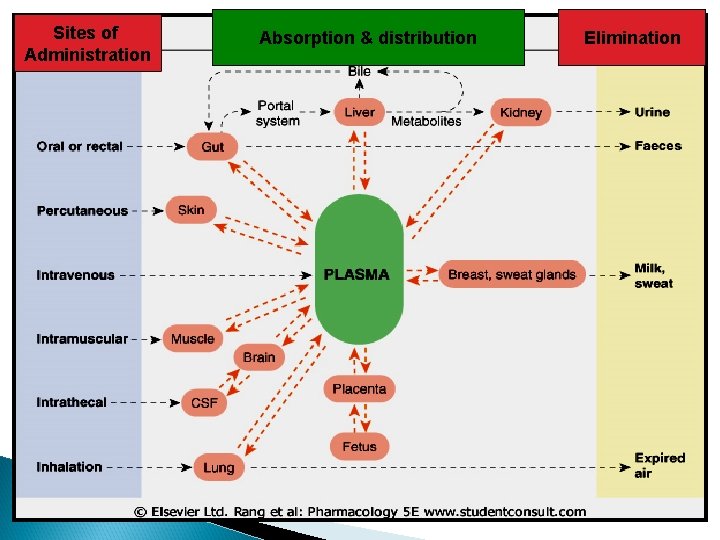
Is the process by which drugs leave blood circulation and enters the interstitium and/or the cells of the tissues.

Sites of Administration Absorption & distribution Elimination

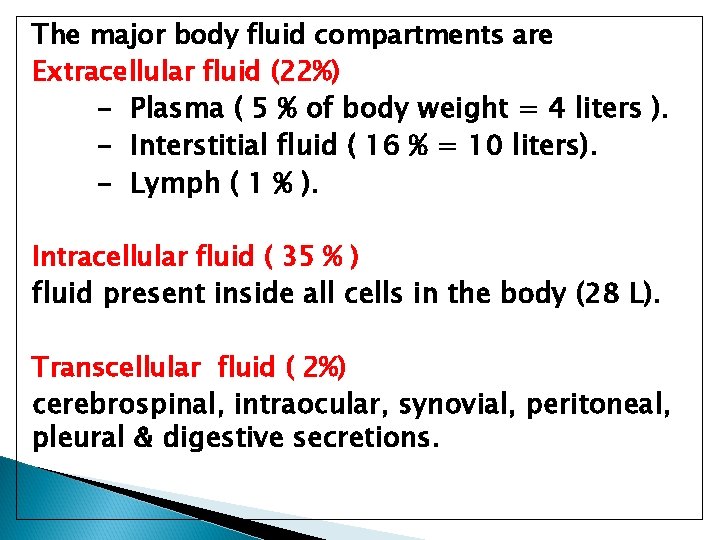
The major body fluid compartments are Extracellular fluid (22%) - Plasma ( 5 % of body weight = 4 liters ). - Interstitial fluid ( 16 % = 10 liters). - Lymph ( 1 % ). Intracellular fluid ( 35 % ) fluid present inside all cells in the body (28 L). Transcellular fluid ( 2%) cerebrospinal, intraocular, synovial, peritoneal, pleural & digestive secretions.

Total body fluids (60% of body weight in 70 -kg individual) Plasma (4 L) Total body Interstitial fluids (10 L) Fluids (42 Liters) Intracellular volume ( 28 L)

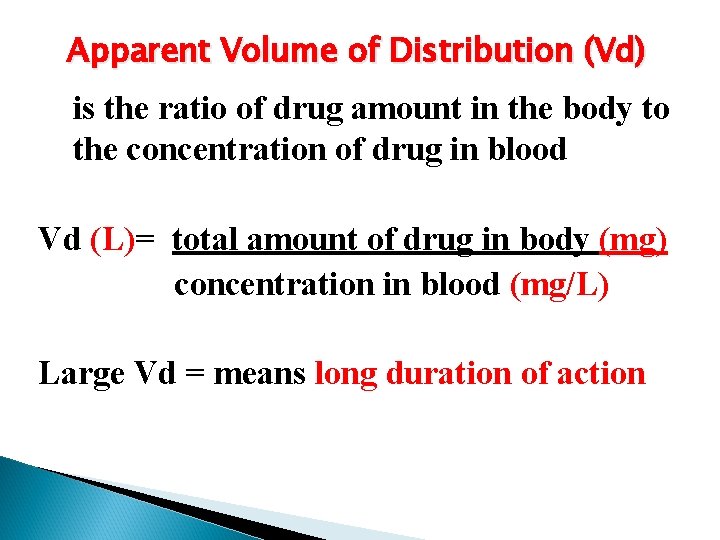
Apparent Volume of Distribution (Vd) is the ratio of drug amount in the body to the concentration of drug in blood Vd (L)= total amount of drug in body (mg) concentration in blood (mg/L) Large Vd = means long duration of action

FACTORS AFFECTING DISTRIBUTION 1. Cardiac output and blood flow. 2. Physiochemical properties of the drug. ◦ Molecular weight ◦ Pka. ◦ Lipid solubility. 3. Capillary Permeability 4. Plasma protein binding 5. Tissue binding.

Blood flow to organs The greater the blood flow to tissues, the more distribution that occurs from plasma to interstitial fluids. Drugs distribute more rapidly to brain, liver and kidney > more than skeletal muscles & fat.

Physiochemical properties Most lipid soluble drugs cross biological membranes Hydrophilic drugs do not readily cross membranes but go through slit junctions

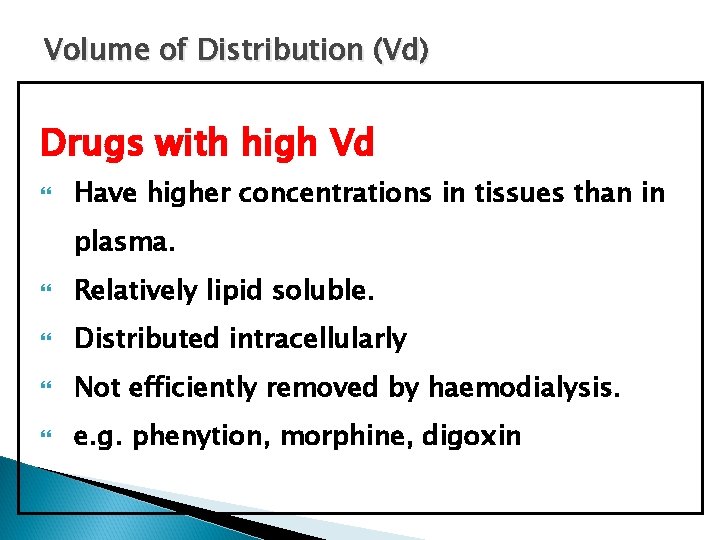
Volume of Distribution (Vd) Drugs with high Vd Have higher concentrations in tissues than in plasma. Relatively lipid soluble. Distributed intracellularly Not efficiently removed by haemodialysis. e. g. phenytion, morphine, digoxin

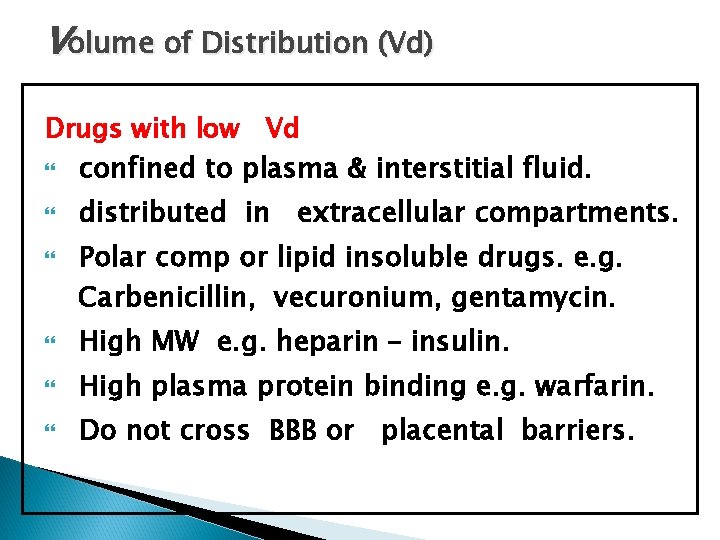
Volume of Distribution (Vd) Drugs with low Vd confined to plasma & interstitial fluid. distributed in extracellular compartments. Polar comp or lipid insoluble drugs. e. g. Carbenicillin, vecuronium, gentamycin. High MW e. g. heparin – insulin. High plasma protein binding e. g. warfarin. Do not cross BBB or placental barriers.

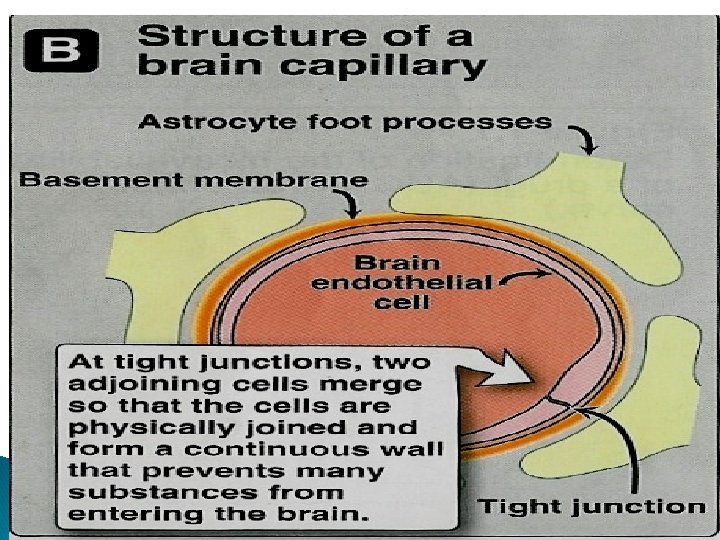
Capillary permeability Endothelial cells of capillaries in tissues other than brain have wide slit junctions allowing easy movement & distribution. Brain has tight junction Blood Brain Barrier (BBB).

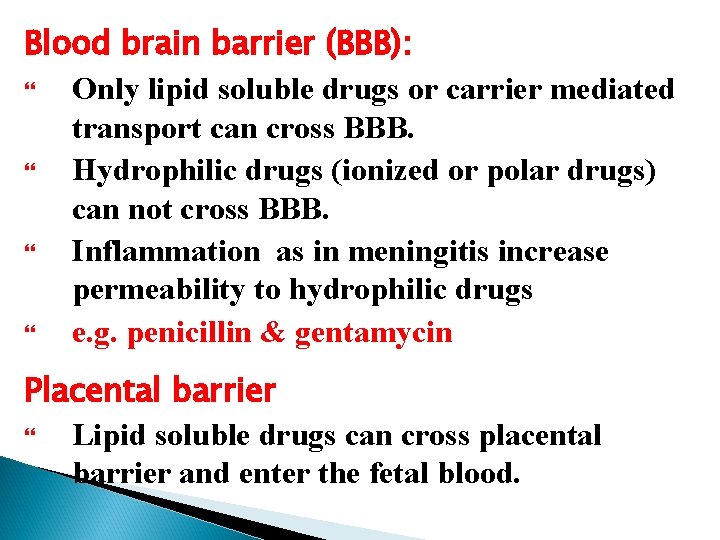
Blood brain barrier (BBB): Only lipid soluble drugs or carrier mediated transport can cross BBB. Hydrophilic drugs (ionized or polar drugs) can not cross BBB. Inflammation as in meningitis increase permeability to hydrophilic drugs e. g. penicillin & gentamycin Placental barrier Lipid soluble drugs can cross placental barrier and enter the fetal blood.





Binding of Drugs ◦ Plasma proteins binding. ◦ Tissue proteins binding.

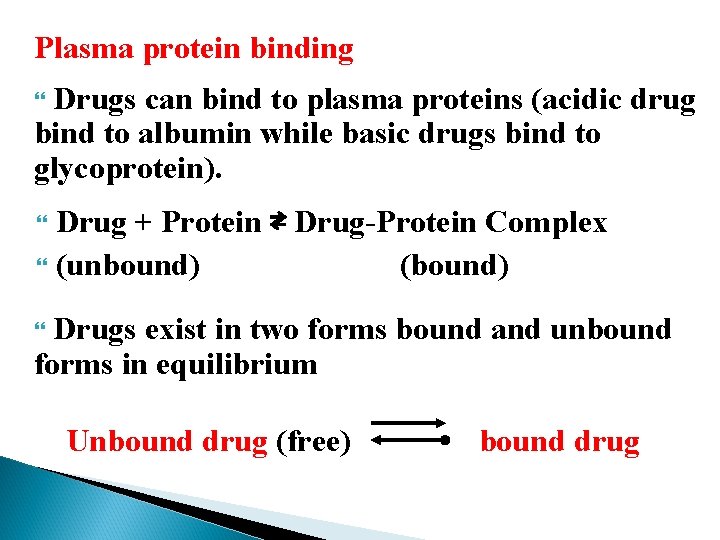
Plasma protein binding Drugs can bind to plasma proteins (acidic drug bind to albumin while basic drugs bind to glycoprotein). Drug + Protein ⇄ Drug-Protein Complex (unbound) (bound) Drugs exist in two forms bound and unbound forms in equilibrium Unbound drug (free) bound drug

bound form of drug § non diffusible form Unbound form of drug § diffusible form § can not combine with § combine with receptors § not available for elimination § has long duration of action (t ½). §available for elimination §has short duration of action (t ½).

Characters & consequences of Binding Usually reversible. determines volume of distribution (vd) Slows drug metabolism & excretion. Prolongs duration of drug action (t 1/2). Result in clinically important drug interactions.

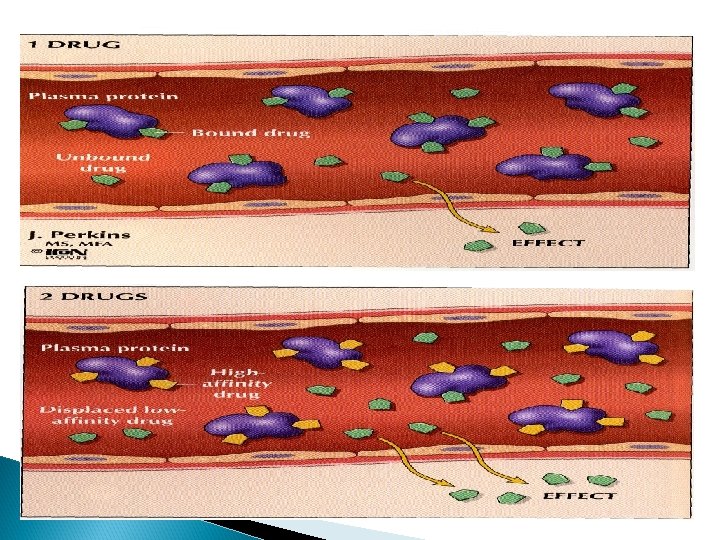
Displacement Competition for the same binding site on the plasma proteins may occur between two drugs displacement of one drug & increasing its "free fraction” and effects. Aspirin + Albumin-warfarin Albumin-aspirin + free warfarin bleeding.

Another example: sulfonamides and bilirubin in a neonates (hyperbilirubinemia).


Tissues Binding Drugs can bind to specific tissue Tetracycline bind to bone Iodides accumulate in salivary & thyroid glands

Redistribution of the drug away from its site of action to other tissues where it can not produce an action e. g. thiopental Termination Ø Biotransformation. Ø Excretion. Ø Redistribution.
 Shiva pakistan
Shiva pakistan A tirinha de hagar estabelece uma interessante contraponto
A tirinha de hagar estabelece uma interessante contraponto Hagar hamed rua
Hagar hamed rua Impedancia normalizada
Impedancia normalizada Drug metabolism definition
Drug metabolism definition Stone angel chapter wise summary
Stone angel chapter wise summary Hagar conjugation
Hagar conjugation Hanan hibshi
Hanan hibshi Hanan habib
Hanan habib Tb meningitis
Tb meningitis Hanan lutfiyya
Hanan lutfiyya How to find a fraction of a fraction
How to find a fraction of a fraction Bilugan ang pangkat ng fraction na magkatumbas
Bilugan ang pangkat ng fraction na magkatumbas Five improper fractions
Five improper fractions Example of like fraction
Example of like fraction Chapter 30 principles of pharmacology
Chapter 30 principles of pharmacology Filtration pharmacology
Filtration pharmacology Mechanism of drug action
Mechanism of drug action Basic & clinical pharmacology
Basic & clinical pharmacology Pharmacology definition
Pharmacology definition Pharmacology for nurses: a pathophysiological approach
Pharmacology for nurses: a pathophysiological approach Focus on pharmacology essentials for health professionals
Focus on pharmacology essentials for health professionals Define pharmacology
Define pharmacology Advantages and disadvantages of drugs
Advantages and disadvantages of drugs Define pharmacology
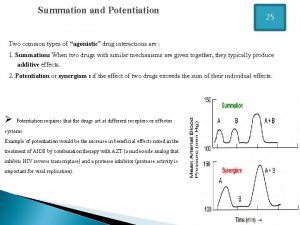
Define pharmacology Potentiation pharmacology example
Potentiation pharmacology example Basic & clinical pharmacology
Basic & clinical pharmacology Pharmacology tutor anderson
Pharmacology tutor anderson Clinical pharmacology powered by clinicalkey
Clinical pharmacology powered by clinicalkey What is pharmacology
What is pharmacology What is ion trapping in pharmacology
What is ion trapping in pharmacology Clinical pharmacology seminar
Clinical pharmacology seminar Efficacy definition pharmacology
Efficacy definition pharmacology