Principles of spasticity management Management of spasticity with



















- Slides: 42

Principles of spasticity management Management of spasticity with botulinum toxin: injection training C. L. I. M. B. Continuum of Learning to Improve Management with Botulinum Toxin DYS-SE-000025 Date of preparation: May 2017 This programme is initiated and funded by IPSEN. Dysport® (Clostridium botulinum type A toxin-haemagglutinin complex) prescribing information is available at this meeting.

Management of spasticity with botulinum toxin: Injection training Introduction: Bo. NT-A formulations Preparation of Dysport® (Abobotulinumtoxin. A, Abo. A) for injection Practical demonstration: Reconstitution Precautions for use Bo. NT-A injection technique Dysport® injection protocol Demonstration and interactive learning Muscle identification techniques Practical considerations for injection

Learning goals • The aim of this module is to: – Review the procedure for injecting botulinum toxin for the treatment of spasticity, including reconstitution and muscle targets, using Dysport® (Abobotulinumtoxin. A, Abo. A) for training purposes – Learn practical injection skills through interactive demonstration, group practice and discussion

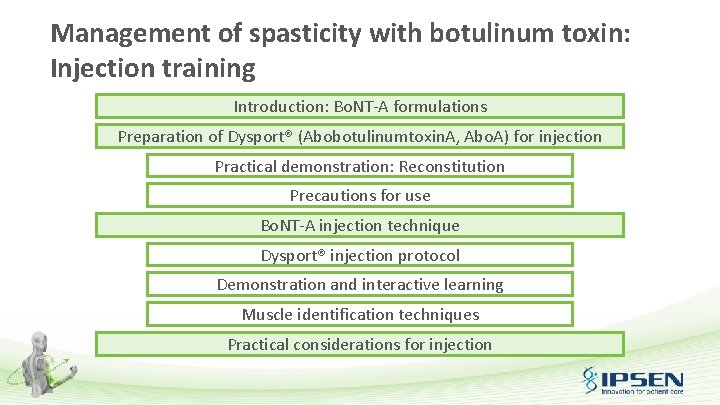
Delete vial sizes if not available in-country Bo. NT-A formulations Dysport® Enterprise Type of toxin Number of units per vial Excipients per vial Ipsen Bo. NT-A Abotulinumtoxin. A 1, 2, 5 Allergan Bo. NT-A Onabotulinumtoxin. A 1, 3, 5 Merz Bo. NT-A Incobotulinumtoxin. A 1, 4, 5 Ipsen Allergan Merz A (+ complex) A 500 U 300 U 200 U, 100 U, 50 U 100 U 50 U HSA: 125 µg Lactose: 2. 5 mg HSA: 500 µg Na. Cl: 0. 9 mg HSA: 1000 µg Sucrose: 4. 7 mg HAS: Human Serum Albumin. 1. FDA press release: www. fda. gov/News. Events/Newsroom/Press. Announcements/2009/ucm 175013. htm (Accessed June 2016); 2. Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); 3. BOTOX® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); 4. XEOMIN® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); 5. Benecke R. Biodrugs 2012; 26(2): e 1 -e 9

For the purposes of this practical injection training session, Dysport® (Bo. NT-A, Ipsen) will be used Preparation of Dysport® (Abobotulinumtoxin. A, Abo. A) for injection Practical demonstration

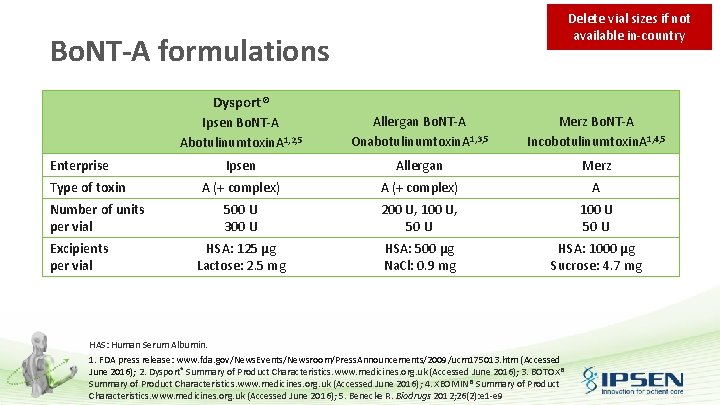
Preparation of Dysport®: Dilution Delete vial sizes if not available in-country • Reconstitution instructions are specific for each of the 300 and 500 U vials • Prior to administration, each 300 U and 500 U vial of lyophilised Dysport® is reconstituted with sodium chloride (Na. Cl) injection B. P. (0. 9%w/v) to yield a final concentration that is specific for the use in each indication Vial Size (U) Diluent Concentration (U/m. L) 300 3. 0 m. L 0. 9% Na. Cl injection BP 100 300 1. 5 m. L 0. 9% Na. Cl injection BP 200 300 0. 6 m. L 0. 9% Na. Cl injection BP 500 5. 0 m. L 0. 9% Na. Cl injection BP 100 500 2. 5 m. L 0. 9% Na. Cl injection BP 200 500 1. 0 m. L 0. 9% Na. Cl injection BP 500 Na. CI: Sodium chloride; B. P. : British Pharmacopeia; w/v: Weight / volume. Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

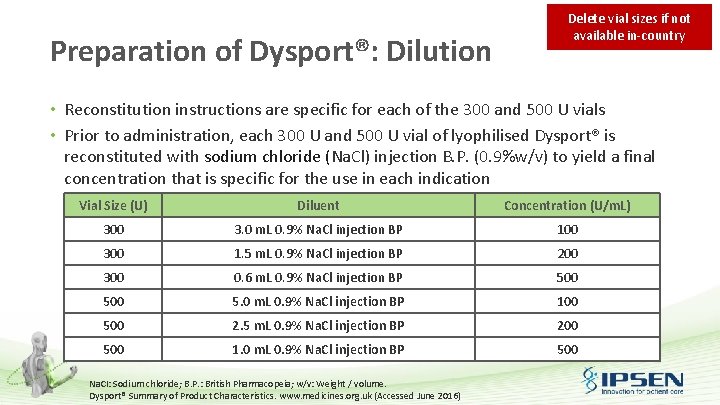
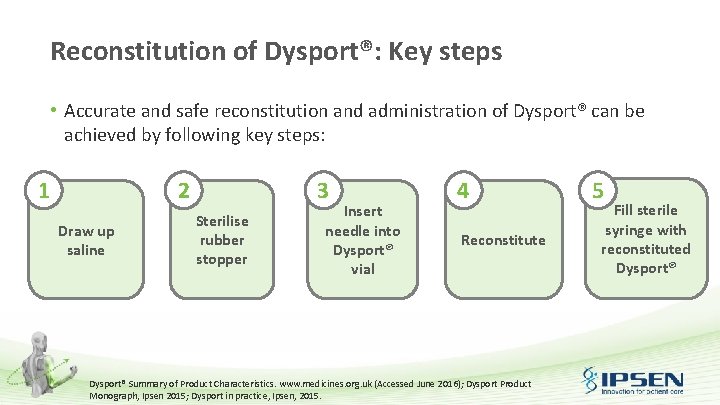
Reconstitution of Dysport®: Key steps • Accurate and safe reconstitution and administration of Dysport® can be achieved by following key steps: 1 2 Draw up saline 3 Sterilise rubber stopper Insert needle into Dysport® vial 4 Reconstitute Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); Dysport Product Monograph, Ipsen 2015; Dysport in practice, Ipsen, 2015. 5 Fill sterile syringe with reconstituted Dysport®

Reconstitution of Dysport®: Key steps 1 • 2 • Draw up saline Draw up the required volume of sterile 0. 9% Na. CI injection BP • A sterile 23 or 25 gauge needle should be used Sterilise rubber stopper The exposed central portion of the rubber stopper should be cleaned with alcohol immediately prior to piercing the septum Na. CI: Sodium chloride; B. P. : British Pharmacopeia. Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); Dysport Product Monograph, Ipsen 2015; Dysport in practice, Ipsen, 2015.

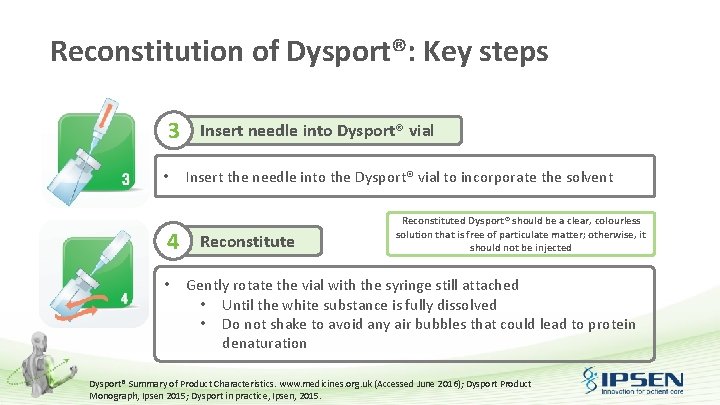
Reconstitution of Dysport®: Key steps 3 • 4 • Insert needle into Dysport® vial Insert the needle into the Dysport® vial to incorporate the solvent Reconstituted Dysport® should be a clear, colourless solution that is free of particulate matter; otherwise, it should not be injected Gently rotate the vial with the syringe still attached • Until the white substance is fully dissolved • Do not shake to avoid any air bubbles that could lead to protein denaturation Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); Dysport Product Monograph, Ipsen 2015; Dysport in practice, Ipsen, 2015.

Reconstitution of Dysport®: Key steps 5 Fill sterile syringe with reconstituted Dysport® • Using the sterile syringe, slowly draw the reconstituted Dysport® solution from the bottom corner of the vial • Do not invert the vial Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); Dysport Product Monograph, Ipsen 2015; Dysport in practice, Ipsen, 2015.

Dysport®: Storage and retention Storage: • Requires refrigeration at temperatures between 2ᴼC and 8ᴼC • Do not freeze Retention: • Unopened vials can be stored for 24 months • Reconstituted solution: – Recommended to be used immediately following reconstitution – Chemical and physical in-use stability has been demonstrated for 24 hours Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

Dysport®: Disposal • Immediately after treatment, any residual Dysport® in the vial or syringe should be inactivated with dilute hypochlorite solution (1% available chlorine) • Any unused product or waste materials should be disposed of in accordance with local requirements • Spillage of Dysport® should be wiped with an absorbent cloth soaked in dilute hypochlorite solution Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

Update this section with local regulations / SMPC Dysport®: precautions for use

Special warnings and precautions for use • Side effects related to the spread of toxin distant from the site of administration have been reported – In some cases these are associated with dysphagia, pneumonia and / or significantly debility, resulting very rarely in death – Patients with therapeutic doses may present with excessive muscle weakness – The risk of occurrence of such undesirable effects may be reduced by using the lowest effective possible dose and by not exceeding the maximum recommended dose • Dysport® should be used with caution and under close medical supervision in patients with subclinical or clinical evidence of marked defective neuromuscular transmission • Dysport® should be administered with caution to patients with pre-existing swallowing or breathing problems as these can worsen following the distribution of the effect of toxin into the relevant muscles Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

Special warnings and precautions for use (cont. ) • Patients and their care-givers must seek immediate medical treatment in case of swallowing, speech or respiratory problems • Dysport® should not be used to treat spasticity in patients who have developed a fixed contracture • As with any intramuscular injection, Dysport® should only be used where strictly necessary in patients with prolonged bleeding times, infection or inflammation at the proposed site(s) of injection • Antibody formation has been noted rarely in patients receiving Dysport® • Careful consideration should be given before the injection of patients who have experienced a previous allergic reaction to a product containing botulinum toxin type A Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

Special warnings and precautions for use (cont. ) • The recommended posology and frequency of administration of Dysport® should not be exceeded • To gain maximum benefit from Dysport®, precautions should be taken in the preparation and administration of the product: – Use Dysport® to treat a single patient during a single session – Discard any residual reconstituted product in accordance with regulations Registrations and advice can differ regionally Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

Drug interactions • The effects of botulinum toxin may be potentiated by drugs interfering either directly or indirectly with neuromuscular function – e. g. aminoglycosides and curare-like non-depolarising blockers • Such drugs should be used with caution in patients treated with botulinum toxin 1 • Non-clinical studies have shown the potential for Bo. NT-A to significantly interact with: 2, 3 • Aminoglycoside antibiotics, guanidine and gangliosides • Guanidine and other potassium channel blockers Registrations and advice can differ regionally 1. Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016); 2. Garcia Sanchez FM, et al. Farmacia Clinica 1992; 9: 44– 53; 3. Adler M, et al. Toxicon 1996; 34: 237– 49.

Pregnancy and lactation • Dysport should be used during pregnancy only if the benefit justifies any potential risk to the foetus. Caution should be exercised when prescribing to pregnant women • It is not known whether Clostridium botulinum type A toxinhaemagglutinin complex is excreted in human milk. The use during lactation cannot be recommended Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed March 2016)

Contraindications • Dysport® is contraindicated in individuals with known hypersensitivity to any of its components: – Clostridium botulinum type A toxin haemagglutinin complex – Human albumin solution – Lactose Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

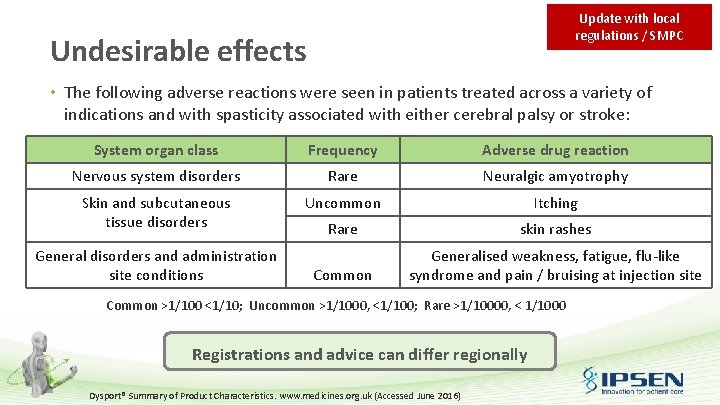
Update with local regulations / SMPC Undesirable effects • The following adverse reactions were seen in patients treated across a variety of indications and with spasticity associated with either cerebral palsy or stroke: System organ class Frequency Adverse drug reaction Nervous system disorders Rare Neuralgic amyotrophy Skin and subcutaneous tissue disorders Uncommon Itching Rare skin rashes Common Generalised weakness, fatigue, flu-like syndrome and pain / bruising at injection site General disorders and administration site conditions Common >1/100 <1/10; Uncommon >1/1000, <1/100; Rare >1/10000, < 1/1000 Registrations and advice can differ regionally Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

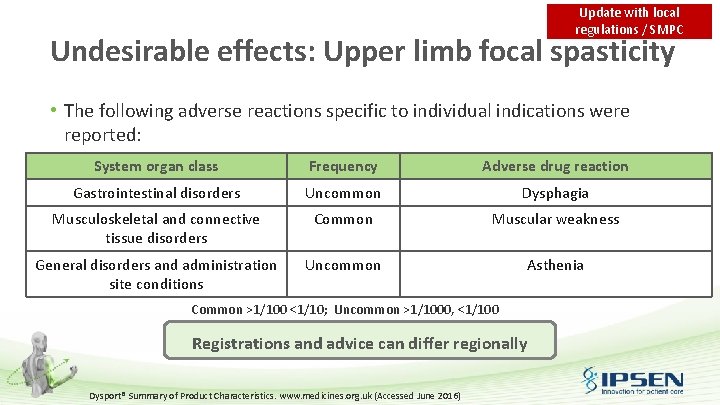
Update with local regulations / SMPC Undesirable effects: Upper limb focal spasticity • The following adverse reactions specific to individual indications were reported: System organ class Frequency Adverse drug reaction Gastrointestinal disorders Uncommon Dysphagia Musculoskeletal and connective tissue disorders Common Muscular weakness General disorders and administration site conditions Uncommon Asthenia Common >1/100 <1/10; Uncommon >1/1000, <1/100 Registrations and advice can differ regionally Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

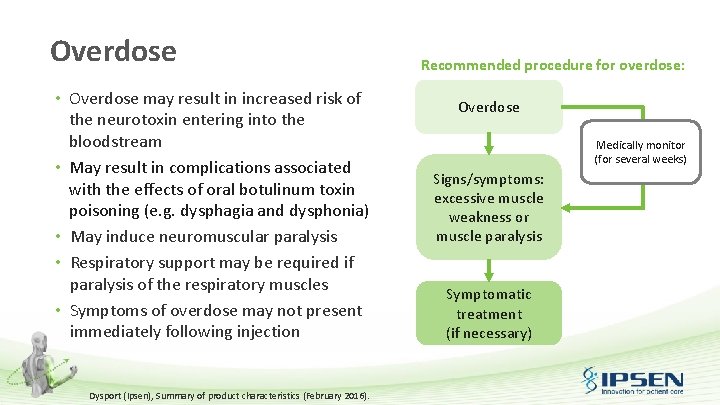
Overdose • Overdose may result in increased risk of the neurotoxin entering into the bloodstream • May result in complications associated with the effects of oral botulinum toxin poisoning (e. g. dysphagia and dysphonia) • May induce neuromuscular paralysis • Respiratory support may be required if paralysis of the respiratory muscles • Symptoms of overdose may not present immediately following injection Dysport (Ipsen), Summary of product characteristics (February 2016). Recommended procedure for overdose: Overdose Medically monitor (for several weeks) Signs/symptoms: excessive muscle weakness or muscle paralysis Symptomatic treatment (if necessary)

Bo. NT-A injection technique: Dysport® injection protocol

Overview of injection technique in spasticity • Dysport® is administered as an intramuscular injection • Although actual location of the injection sites can be determined by palpation the use of injection guiding technique (e. g. EMG, ES or US) is recommended to target the injection sites • Clinical improvement may be expected one week after injection and may last up to 20 weeks • Injections can be repeated every 12– 16 weeks; more frequent injections are not recommended Injection technique is dependent on indications licensed in your country / region Please refer to your local summary of product characteristics and guidelines EMG: Electromyography; ES: Electrical stimulation; US: Ultrasound. Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

Injection sites: Upper limb Countries to adapt to local licence • Dosing in initial and sequential treatment sessions should be tailored to the individual based on: – – – Size Number and location of muscles involved Severity of spasticity Presence of local muscle weakness Patient's response to previous treatment, and/or adverse event history with Dysport® • In clinical trials, doses of 500 Units and 1000 Units were divided among selected muscles at a given treatment session as shown on the next slide • No more than 1 m. L should generally be administered at any single injection site Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

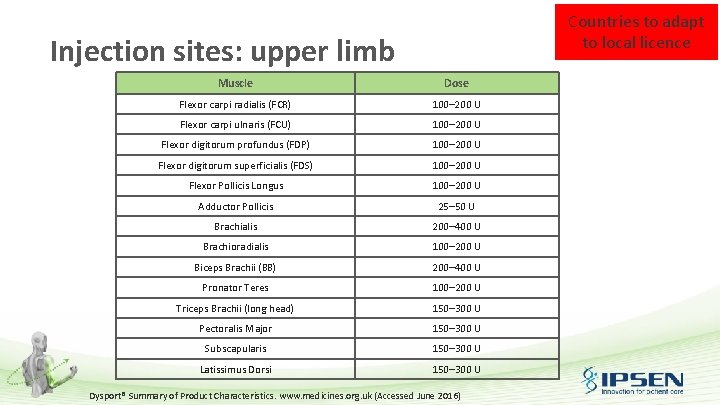
Countries to adapt to local licence Injection sites: upper limb Muscle Dose Flexor carpi radialis (FCR) 100– 200 U Flexor carpi ulnaris (FCU) 100– 200 U Flexor digitorum profundus (FDP) 100– 200 U Flexor digitorum superficialis (FDS) 100– 200 U Flexor Pollicis Longus 100– 200 U Adductor Pollicis 25– 50 U Brachialis 200– 400 U Brachioradialis 100– 200 U Biceps Brachii (BB) 200– 400 U Pronator Teres 100– 200 U Triceps Brachii (long head) 150– 300 U Pectoralis Major 150– 300 U Subscapularis 150– 300 U Latissimus Dorsi 150– 300 U Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

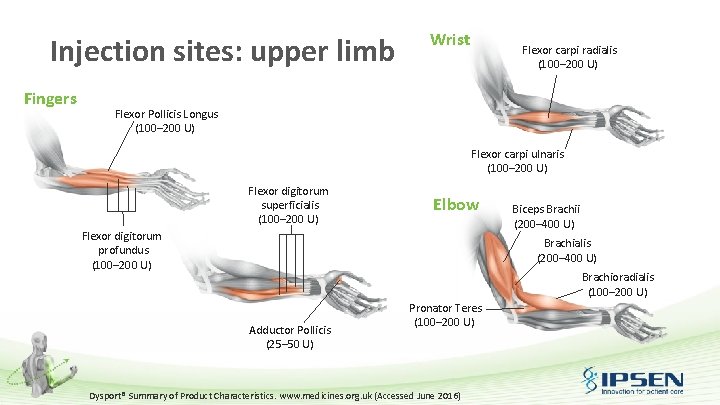
Injection sites: upper limb Fingers Wrist Flexor carpi radialis (100– 200 U) Flexor Pollicis Longus (100– 200 U) Flexor carpi ulnaris (100– 200 U) Flexor digitorum superficialis (100– 200 U) Elbow Flexor digitorum profundus (100– 200 U) Biceps Brachii (200– 400 U) Brachialis (200– 400 U) Brachioradialis (100– 200 U) Adductor Pollicis (25– 50 U) Pronator Teres (100– 200 U) Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

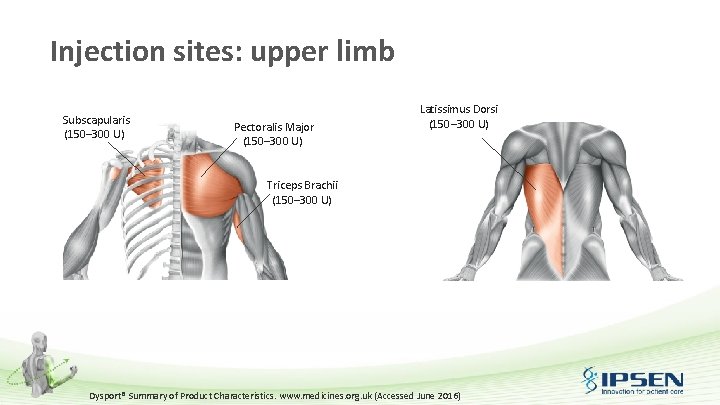
Injection sites: upper limb Subscapularis (150– 300 U) Pectoralis Major (150– 300 U) Latissimus Dorsi (150– 300 U) Triceps Brachii (150– 300 U) Dysport® Summary of Product Characteristics. www. medicines. org. uk (Accessed June 2016)

Injection sites: lower limb Countries to adapt to local licence • Depending on the cases, the exact dose and the number of injection sites will generally be adjusted, according to: – – – Size Number and location of the muscles affected Severity of the spasticity Presence of muscular weakness Response to the previous treatment • Recommended dose is 1500 units, distributed between the gastrocnemius and soleus muscles although injections of the tibialis posterior should also be considered Dysport® Summary of Product Characteristics, France; Dysport in practice, Ipsen, 2015.

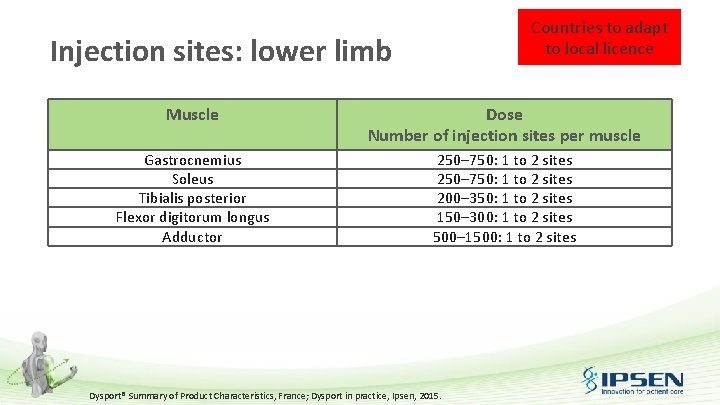
Countries to adapt to local licence Injection sites: lower limb Muscle Dose Number of injection sites per muscle Gastrocnemius Soleus Tibialis posterior Flexor digitorum longus Adductor 250– 750: 1 to 2 sites 200– 350: 1 to 2 sites 150– 300: 1 to 2 sites 500– 1500: 1 to 2 sites Dysport® Summary of Product Characteristics, France; Dysport in practice, Ipsen, 2015.

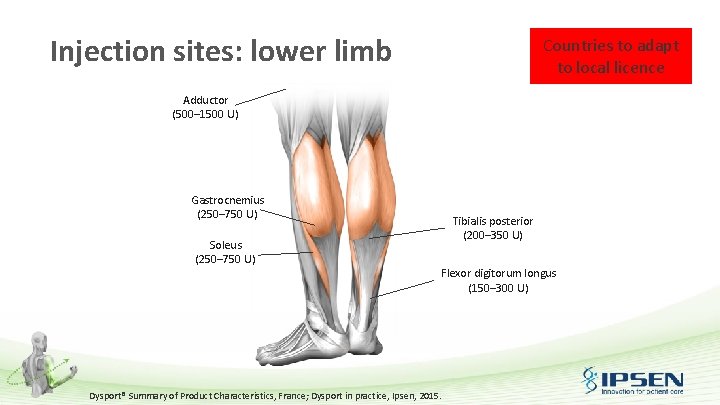
Injection sites: lower limb Countries to adapt to local licence Adductor (500– 1500 U) Gastrocnemius (250– 750 U) Soleus (250– 750 U) Tibialis posterior (200– 350 U) Flexor digitorum longus (150– 300 U) Dysport® Summary of Product Characteristics, France; Dysport in practice, Ipsen, 2015.

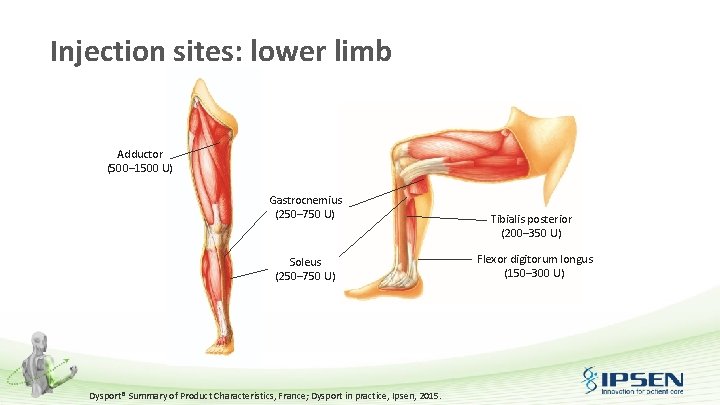
Injection sites: lower limb Adductor (500– 1500 U) Gastrocnemius (250– 750 U) Soleus (250– 750 U) Dysport® Summary of Product Characteristics, France; Dysport in practice, Ipsen, 2015. Tibialis posterior (200– 350 U) Flexor digitorum longus (150– 300 U)

Dysport® injection technique: demonstration and interactive learning

Muscle identification techniques

Muscle identification techniques • Planning and siting of injections should be undertaken by the clinician and multi-professional team 1, 2 • Larger, superficial muscles may be identified with knowledge of anatomy 1, 2 – These techniques include observation, identification of bony landmarks, palpation, and muscle activation and relaxation 2 • Smaller, less accessible muscles may require additional techniques to ensure correct placement of the injection 1, 2 1. Turner-Stokes L, Ward AB, et al. National guidelines. RCP and BSRM, 2009; 2. Elovic EP, et al. PM R 2009; 1: 842– 51.

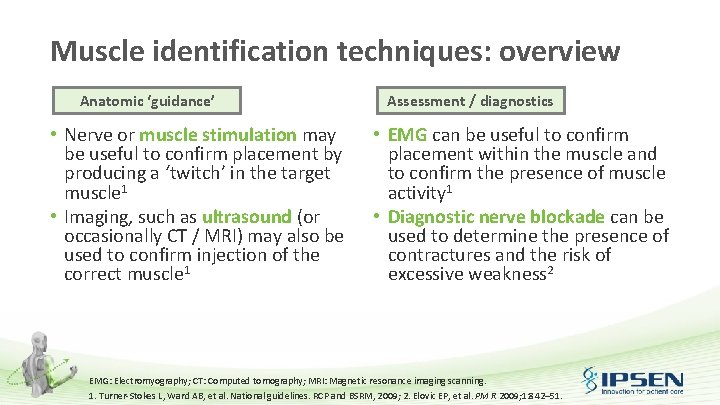
Muscle identification techniques: overview Anatomic ‘guidance’ • Nerve or muscle stimulation may be useful to confirm placement by producing a ‘twitch’ in the target muscle 1 • Imaging, such as ultrasound (or occasionally CT / MRI) may also be used to confirm injection of the correct muscle 1 Assessment / diagnostics • EMG can be useful to confirm placement within the muscle and to confirm the presence of muscle activity 1 • Diagnostic nerve blockade can be used to determine the presence of contractures and the risk of excessive weakness 2 EMG: Electromyography; CT: Computed tomography; MRI: Magnetic resonance imaging scanning. 1. Turner-Stokes L, Ward AB, et al. National guidelines. RCP and BSRM, 2009; 2. Elovic EP, et al. PM R 2009; 1: 842– 51.

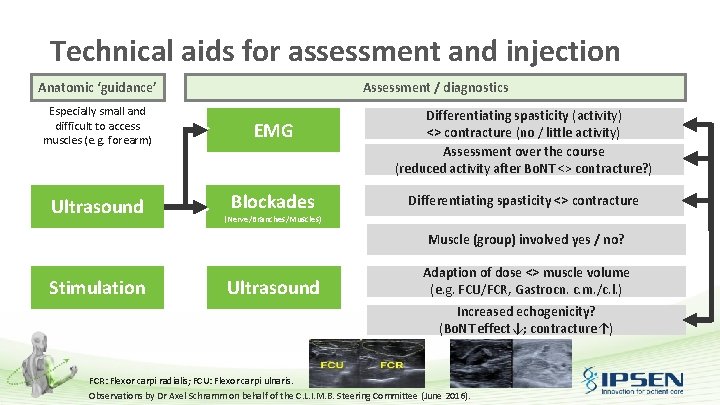
Technical aids for assessment and injection Anatomic ‘guidance’ Assessment / diagnostics Especially small and difficult to access muscles (e. g. forearm) EMG Ultrasound Blockades Differentiating spasticity (activity) <> contracture (no / little activity) Assessment over the course (reduced activity after Bo. NT <> contracture? ) Differentiating spasticity <> contracture (Nerve/Branches/Muscles) Muscle (group) involved yes / no? Stimulation Ultrasound Adaption of dose <> muscle volume (e. g. FCU/FCR, Gastrocn. c. m. /c. l. ) Increased echogenicity? (Bo. NT effect↓; contracture↑) FCR: Flexor carpi radialis; FCU: Flexor carpi ulnaris. Observations by Dr Axel Schramm on behalf of the C. L. I. M. B. Steering Committee (June 2016).

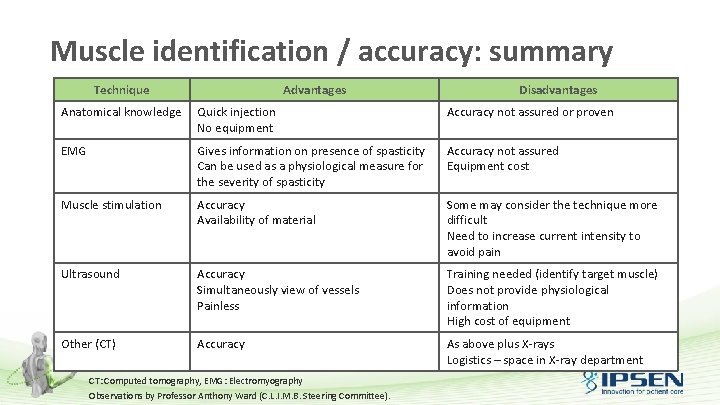
Muscle identification / accuracy: summary Technique Advantages Disadvantages Anatomical knowledge Quick injection No equipment Accuracy not assured or proven EMG Gives information on presence of spasticity Can be used as a physiological measure for the severity of spasticity Accuracy not assured Equipment cost Muscle stimulation Accuracy Availability of material Some may consider the technique more difficult Need to increase current intensity to avoid pain Ultrasound Accuracy Simultaneously view of vessels Painless Training needed (identify target muscle) Does not provide physiological information High cost of equipment Other (CT) Accuracy As above plus X-rays Logistics – space in X-ray department CT: Computed tomography, EMG: Electromyography Observations by Professor Anthony Ward (C. L. I. M. B. Steering Committee).

Practical considerations for injection with Bo. NT-A: group discussion

Practical considerations: group discussion • As a group, discuss the following points considering day-to-day clinical practice: – Who from the service team injects botulinum toxin (e. g. clinician, physiotherapist)? – Who conducts the pre-injection checks (i. e. contraindications)? – Who prepares the syringe? – How in the treatment room organised (materials and equipment)? – What type of needle do you use? – Which muscle should you inject first? – Who oversees aseptic preparations?

Practical considerations: group discussion • As a group, discuss the following points considering day-to-day clinical practice: – How is analgesia managed (type of treatment)? – What information is given to patients and when? – What is the expected responses from patients? – How and when are outcomes measured? – Scheduling of next injection appointment? – How are outcomes optimised (treatment individualisation)?

Abbreviated prescribing information <<<Local abbreviated prescribing information for Dysport® to be inserted>>
 Ashworth tone scale
Ashworth tone scale Spasticity vs tone
Spasticity vs tone Spasticity vs tone
Spasticity vs tone Pramopexole
Pramopexole Hypertonia vs spasticity
Hypertonia vs spasticity Clasp knife rigidity
Clasp knife rigidity Hemianopasia
Hemianopasia Ashworth tone scale
Ashworth tone scale Corticobulbar tract
Corticobulbar tract Spasticity
Spasticity Spasticity
Spasticity Spasticity
Spasticity Spasm
Spasm Non compressive myelopathy
Non compressive myelopathy Basic principles of cost management
Basic principles of cost management Pearson unit 6 principles of management
Pearson unit 6 principles of management Total quality management 14 points
Total quality management 14 points Five (5) principles of islamic management.
Five (5) principles of islamic management. Toyota way: 14 principles
Toyota way: 14 principles What are the principles of stakeholder management
What are the principles of stakeholder management Principles of waste management
Principles of waste management Project management practices and principles
Project management practices and principles Which two principles of channel distribution
Which two principles of channel distribution Henry ford principles of management
Henry ford principles of management Father of management henri fayol
Father of management henri fayol Principle of information system
Principle of information system Material management concept
Material management concept Principles of management by stephen p robbins
Principles of management by stephen p robbins What are disasters
What are disasters 7 principles of total quality management
7 principles of total quality management Principles of cash management
Principles of cash management Unit 6 principles of management sample answers
Unit 6 principles of management sample answers Statement of principles of trust department management
Statement of principles of trust department management Principles of marketing by philip kotler
Principles of marketing by philip kotler Project portfolio management guiding principles
Project portfolio management guiding principles Gamg plank
Gamg plank Energy management principles
Energy management principles Mgmt 11 principles of management
Mgmt 11 principles of management Toyota 14 management principles
Toyota 14 management principles Project management principles and practices
Project management principles and practices What are the three principles of outbreak management
What are the three principles of outbreak management Change management principles and practices
Change management principles and practices The father of scientific management?
The father of scientific management?



























































