Spasticity Management in Neurological Conditions A QuickStart Guide



































- Slides: 45

Spasticity Management in Neurological Conditions A Quick-Start Guide George F. Wittenberg MD Ph. D 28 January 2010

Outline • The Upper Motor Neuron Syndrome • Spasticity Management through Oral Medications • Spasticity Management through Parenteral Medications – Botulinum Toxins – Intrathecal Baclofen • Emergency Management

Upper Motor Neuron Syndrome Positive Signs (Excessive normal resting state) – Spasticity – Rigidity – Hyperreflexia – Primitive reflexes – Clonus Negative Signs (Less than normal resting state) – Lack of strength – Lack of motor control – Lack of coordination Young RR, Emre M, Nance PW, et al. Current issues in spasticity management. Neurologist. 1997; 3: 261275. Young RR. Treatment of spastic paresis. N Engl J Med. June 1989; 320(23): 1553 -1555.

Examples of UMN Lesions – Stroke – Multiple Sclerosis – Corticobasal Ganglionic Degeneration – Traumatic Brain Injury – Acquired Brain Injury – Spinal Cord Injury – Cerebral Palsy

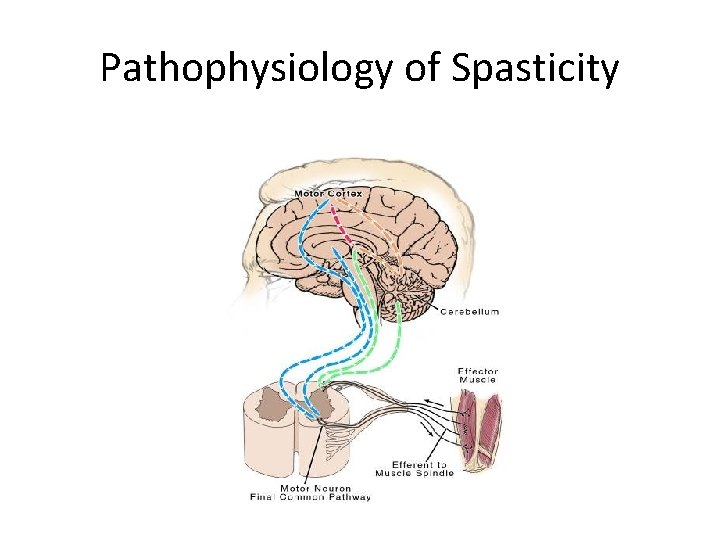
Pathophysiology of Spasticity

Spasticity: Consequences • Pain and discomfort • Contractures • Increased energy cost of movement • Skin breakdown–shear • Interferes with breathing • Hampers gait and transfers • • • Interferes with hygiene More work for caregiver Poor safety Sexual difficulties Insomnia Poor posture

Spasticity: Assessment Patient report Deep tendon reflexes Passive range of motion Test for clonus Functional observation – Determine nature of hypertonicity – Assess interference with function (gait) and effect of stress and/or fatigue – Assess adaptive shortening of muscles vs. irreversible contracture • Consider aggravating factors (e. g. , UTI, infection, excessive activity, strengthening exercises) • • •

Modified Ashworth Scale 0. No increase in muscle tone 1. (1) Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension. 2. (1+) Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the reminder (less than half) of the ROM (range of movement). 3. (2) More marked increase in muscle tone through most of the ROM, but affected part(s) easily moved. 4. (3) Considerable increase in muscle tone passive, movement difficult. 5. (4) Affected part(s) rigid in flexion or extension. (Original Ashworth score in parentheses; Modified after Bohannon and Smith Phys Ther. 1987 Feb; 67(2): 206 -7.

Considerations in Reducing Spasticity • Possible Advantages of Spasticity – Maintains muscle tone/bulk – Helps support circulatory function – May prevent formation of deep vein blood thrombosis – May assist in activities of daily living – May assist with postural control

Spasticity: Rehabilitation Intervention • Static stretch • Gait training (emphasis • • on swing and heel strike) Positioning/posture Cooling Patient education Relaxation • Bio-feedback • Reflex-inhibiting • • movement patterns Avoiding noxious stimuli Medications ITB Therapy Botulinum Toxin

Spasticity: Medication • Baclofen (Lioresal) – GABA agonist – Max. dose 180 mg daily, divided – Side effects: sedation, incontinence, weakness, withdrawal seizures • Tizanidine (Zanaflex) – Alpha 2 adrenergic agonist – Max. dose 24 -32 mg daily – Side effects: sedation, hypotension, weakness, hepatotoxicity • Dantrolene - reserve for specialists • Diazepam et al. (Valium) • Gabapentin (Neurontin)

Considerations in Starting Oral Medications • Titrate slowly: increase dose every five days, starting at: – 5 mg baclofen q. HS – 2 mg tizanidine q. HS • Inform patients to back off to previous dose if they encounter problems with sedation, other expected effects. • Effectiveness variable, often marginal

Spasticity: Invasive ℞ • Botulinum Toxins A & B • Baclofen pump – Intrathecal delivery via implanted pump (ITB) • Surgical interventions – Tenotomy (Tendon Lengthening) – Selective Rhizotomy

Botulinum Toxins • Derived from Clostridium botulinum bacteria • Multiple forms, but only A & B clinically approved • Produce reduction in muscle overactivity from 2 -200 days after injection – Peak effect begins 7 -10 days after – Should not be reinjected before 90 days – May eventually become ineffective due to antibodies

Injection Procedure • Injected near motor points of muscles • Multiple injections per muscle • Monitor with EMG (optional) • Allows identification of muscles • Can help identify best targets

Common Spasticity syndromes treatable with Botulinum Toxin • Thumb in palm – and clenched hand/flexed wrist in general • Elbow flexion • Striatal/Clenched toes • Equinovarus deformity • Scissoring Gait

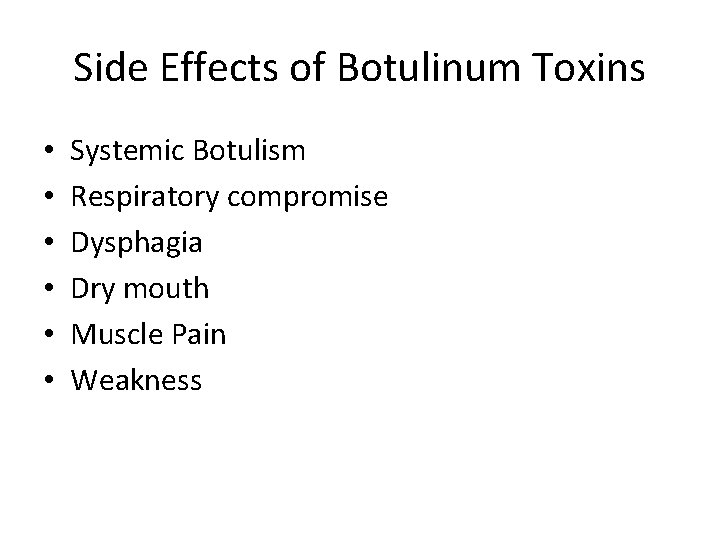
Side Effects of Botulinum Toxins • • • Systemic Botulism Respiratory compromise Dysphagia Dry mouth Muscle Pain Weakness

Response Problems • Poor response may be primary (with first injection) or secondary • Reasons for poor response – – – Poor muscle injection technique Incorrenct muscle selection Dosing inadequate Muscle involvement has changed �Soft tissue contracture • Neutralizing antibodies may be present (but rare in spasticity) – Tests for nonresponse: frontalis test, – antibody assays (limited sensitivity, specificity)

Dosing of Botulinum Toxin • Botox (onabotulinumtoxin. A) available in 100 unit vials of lyophilized powder – Max dose not established, but FDA warns against dosing more than 400 units • Myo. Bloc (rimabotulinumtoxin. B) units are about 50 x less potent than Botox • So 100 units Botox are approximately equivalent to 5000 units Myo. Bloc

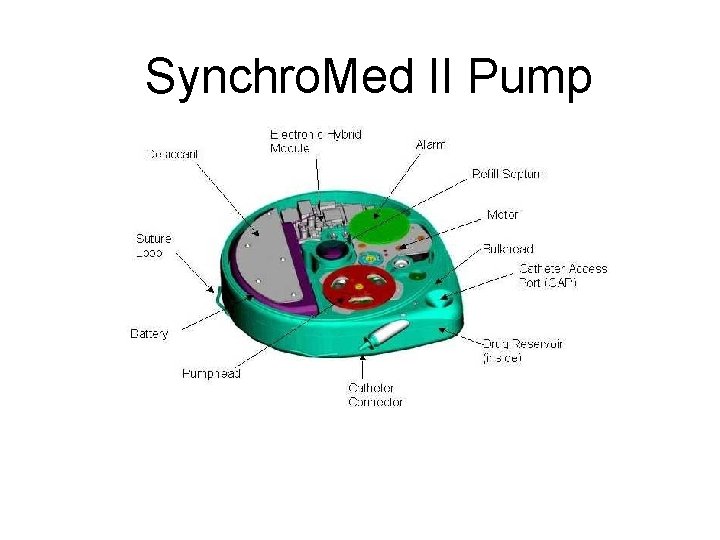
ITB Therapy • Intrathecal Baclofen Therapy delivers liquid baclofen directly into the intrathecal space around the spinal cord • Drug delivered via an implanted and programmable pump connected to a catheter • Approved by FDA in 1992 for spinal origin spasticity and 1996 for cerebral origin spasticity. – Components are completely implanted (except the programmer) – Programmer is used to adjust the pump. – Implanting the pump is the beginning of therapy, takes 30 -90 days to adjust dosage

Synchro. Med II Pump

Pump Compatibility • The following are unlikely to affect pump operation or damage the pump: – Electrocautery – Diagnostic Ultrasound – Low-Power Therapeutic Ultrasound – i. e. the type used in P. T. – Pacemakers/ICD’s – Diagnostic X-rays – TENS – Laser Procedures – Pressurized Aircraft – Theft detectors/Security Devices – Home Appliances – Tanning Bed

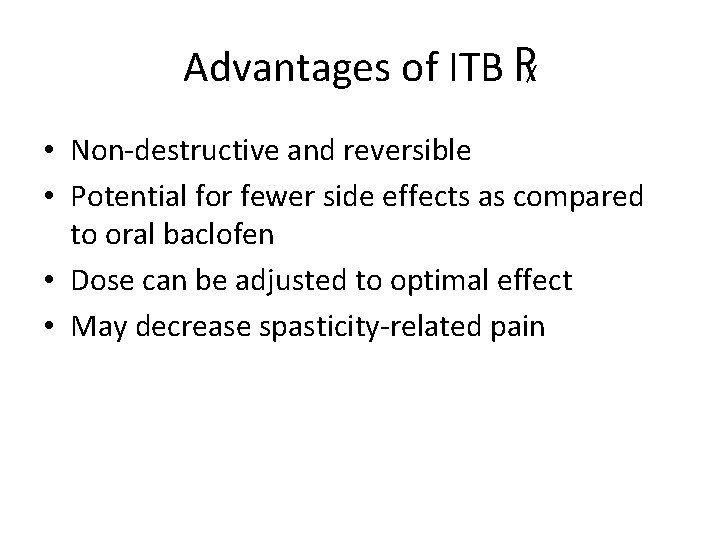
Advantages of ITB ℞ • Non-destructive and reversible • Potential for fewer side effects as compared to oral baclofen • Dose can be adjusted to optimal effect • May decrease spasticity-related pain

Potential Risks of ITB ℞ • Most common side effects: weakness, drowsiness, nausea/vomiting, headache, and dizziness • Overdose, although rare, could lead to respiratory depression, loss of consciousness, coma, and in extreme cases, may be life-threatening • Infection

Potential Risks of ITB ℞ • Abrupt discontinuation can result in high fever, altered mental status, worsened spasticity, and muscle rigidity – needs to be treated as life threatening • Causes: – Empty pump reservoir – Catheter failure: disconnecting from pump, kinking, migrating or breaking – Electromechanical failure, e. g. battery failure

Management Issues: Spasticity has increased – Patient not getting enough drug • Low reservoir • Programming/refill error • Pump or catheter problem – Factor causing increase in spasticity • Disease progression • Physiologic response

Management Issues: Spasticity has decreased – hypotonia – Patient getting too much drug • • • Programming error Refill error Subdural catheter Pump pocket injection Oral medication

Management Issues: Altered Mental Status • Altered mental status – Could be sign of overdose or withdrawal • Has spasticity increased or decreased? • Other associated symptoms

Circumstances That May Require Added Attention Higher ITB Therapy doses Non-verbal patient Inability to identify problem/localize pain ITB Therapy naïve medical system – ER/ICU doesn’t contact “pump doctor” until after cascade effect • After pump replacement • After pump removal for infection • •

Lioresal Intrathecal • Lioresal Intrathecal is an analog to the naturally occurring inhibitory neurotransmitter gamma-aminobutyric acid (GABA) – Effects similar to benzodiazepines • Synchro. Med® II pump reservoir holds 20 or 40 ml of drug – 6 -month supply • 500 mcg/ml • 2000 mcg/ml

Overdose • Symptoms Are Dose-Dependent – Mild • Hypotonia • Difficulty concentrating • Progressive decrease in tone to flaccid – Moderate • Somnolence • Obtundation • Bradycardia – Severe • Stupor • Hypoventilation to apnea • Coma

Overdose • Iatrogenic – Likely due to human error – Programming error – Unanticipated effect of dosage or concentration • Synchro. Med pump vs. catheter malfunction • Difficult to ascertain cause

Overdose – Mechanical Causes • Subdural catheter • Filling of pump pocket – – 18 ml of Lioresal Intrathecal 500 mcg/ml=9 mg 18 ml of Lioresal Intrathecal 2000 mcg/ml=36 mg 40 ml of Lioresal Intrathecal 500 mcg/ml=20 mg 40 ml of Lioresal Intrathecal 2000 mcg/ml=80 mg

Overdose - Treatment Suggested Treatments • Mild – Decrease dose • Moderate – Stop pump for several hours (2 -4 hours) • Program minimal infusion bolus, then restart at lower dose – Decrease dose • Monitor patient carefully for at least 24 hours

Severe Overdose Suggested Treatment • Maintain airway/breathing/circulation • Empty pump reservoir to stop drug flow – Aspirate reservoir if no programmer – Remember to schedule restart • Administer physostigmine/pressors – +/- physostigmine(0. 5 -1. 0 mg increments IV) • LP to withdraw 30 – 40 m. L CSF • Notify patient's ITB Therapy physician • Continue to monitor closely for symptom recurrence

Suggested Treatment • Remove CSF – An effective treatment • Effective in first 4 hours • Still may be effective up to 8 hours – Catheter access port (CAP) – 30 – 40 m. L – Lumbar puncture if cannot aspirate CAP

Sequelae of Severe Overdose • Patient – Less frequent if airway is protected and hypotension treated rapidly • Family – Fear of patient’s death – Fear of brain damage – Intensive emotional support needed

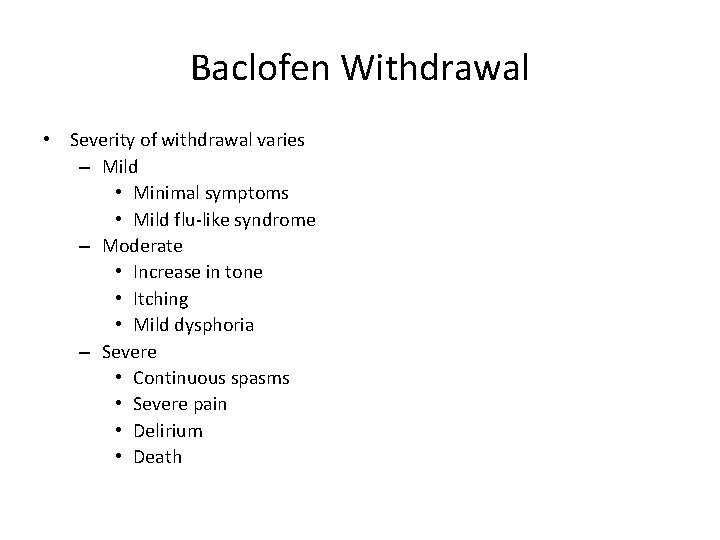
Baclofen Withdrawal • Severity of withdrawal varies – Mild • Minimal symptoms • Mild flu-like syndrome – Moderate • Increase in tone • Itching • Mild dysphoria – Severe • Continuous spasms • Severe pain • Delirium • Death

Symptoms of Underdose • • • Pruritis without rash Hypotension Paresthesias Fever Altered mental status

Symptoms of Withdrawal • • Exaggerated rebound spasticity and muscle rigidity Might be diagnosed in ER as seizures Rhabdomyolysis Multiple organ failure May resemble • Autonomic dysreflexia • Sepsis • Malignant hyperthermia • Neuroleptic-malignant syndrome

Suggested Treatment Always assume it’s the Intrathecal Baclofen • Initiate life-sustaining measures if indicated • Thorough history – Abrupt vs insidious onset – Presence of VPS – Recent refill/replacement/programming • Start oral baclofen – Each patient needs prescription at home – Remind family to take prescription on vacation

Other Interventions – Benzodiazepines • GABA agonists • IV or PO – Cyproheptadine (serotonin antagonist)* • Cyproheptadine 4 -8 mg every 6 hours – Lioresal Intrathecal via LP • 50 -100 mcg bolus – Lumbar infusion via bedside pump and percutaneous catheter • Maintenance • Weaning ITB Therapy *Meythaler JM, Roper JF, Brunner RC. Cyproheptadine for intrathecal baclofen withdrawal. Arch Phys Med Rehabil 2003; 84: 638 -642.

Pearls to Prevent Withdrawal Refill pump if suspect low reservoir Compare actual vs expected aspirate Verify concentration of last refill Systems to ensure patients receive refills in a timely manner • Examine programming after pump replacement • Troubleshoot pump and catheter immediately upon suspicion of malfunction • Patients should have some oral baclofen available for emergency • •

Conclusions • While spasticity management can be difficult, it may also improve patient’s quality of lift • Spasticity is not necessarily the enemy, but is part of a pattern of abnormal motor control • Choice of treatment depends on pattern of involvement

Contact • For questions about this audio conference please contact Dr. George Wittenberg at george. wittenberg@va. gov • For any questions about the monthly GRECC Audio Conference Series please contact Tim Foley at tim. foley@va. gov or call (734) 222 -4328 • To evaluate this conference for CE credit please obtain a ‘Satellite Registration’ form and a ‘Faculty Evaluation’ form from the Satellite Coordinator at you facility. The forms must be mailed to EES within 2 weeks of the broadcast