Polymer Properties Exercise 1 Structure Draw the different
![3) • Calculate the required viscosity parameters: c efflux time [mg/ml] [t/s] r = 3) • Calculate the required viscosity parameters: c efflux time [mg/ml] [t/s] r =](https://slidetodoc.com/presentation_image_h/89342a01de98935b7987db60dbe220cd/image-15.jpg)
![3) • [ ] is obtained from the plot from the crossing of y-axis: 3) • [ ] is obtained from the plot from the crossing of y-axis:](https://slidetodoc.com/presentation_image_h/89342a01de98935b7987db60dbe220cd/image-16.jpg)
- Slides: 18

Polymer Properties Exercise 1

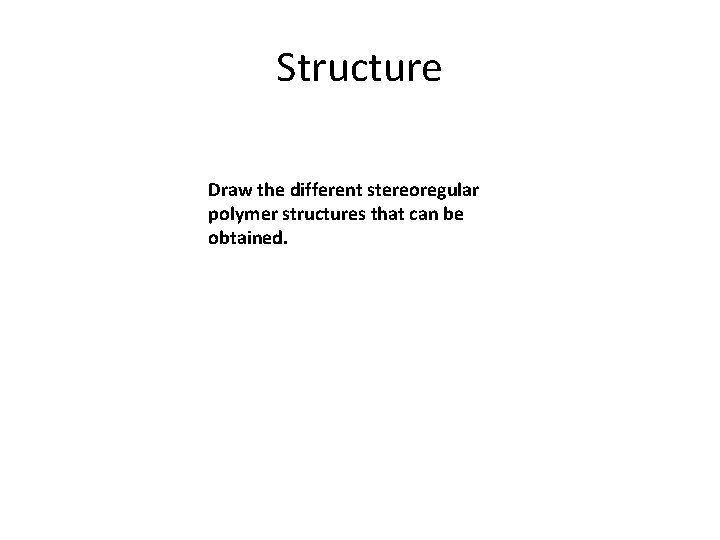
Structure Draw the different stereoregular polymer structures that can be obtained.

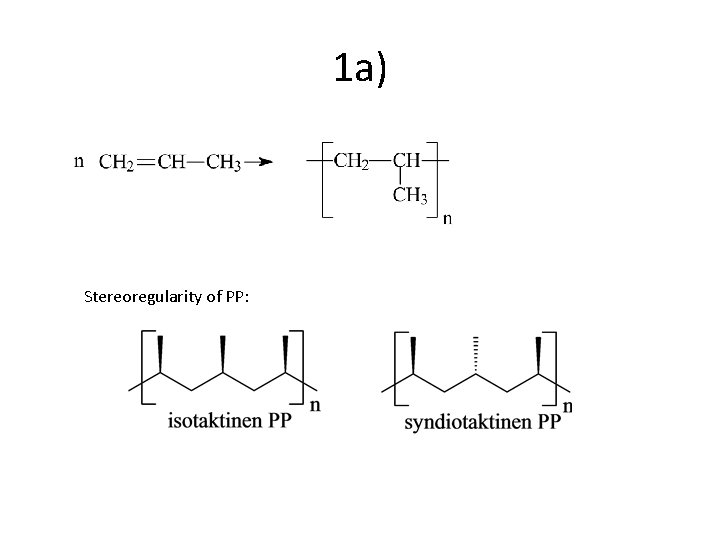
1 a) Stereoregularity of PP:

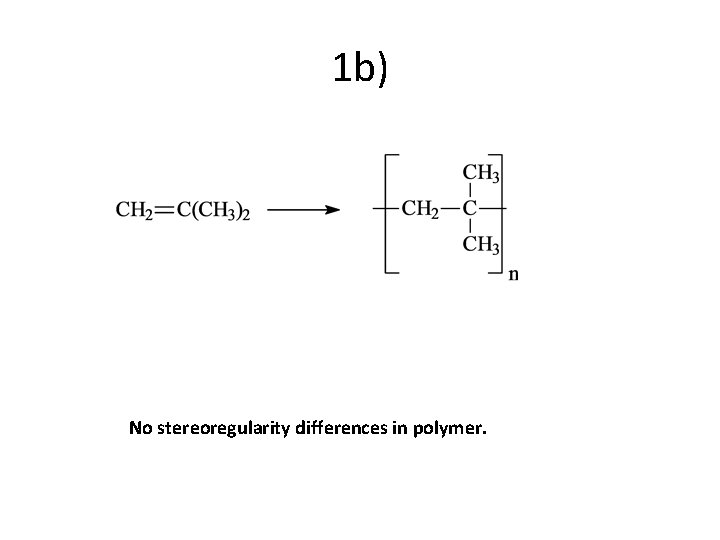
1 b) No stereoregularity differences in polymer.

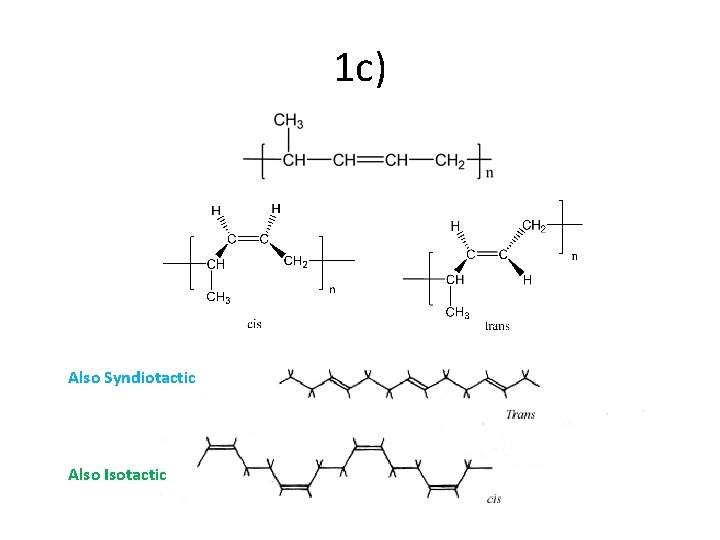
1 c) Also Syndiotactic Also Isotactic

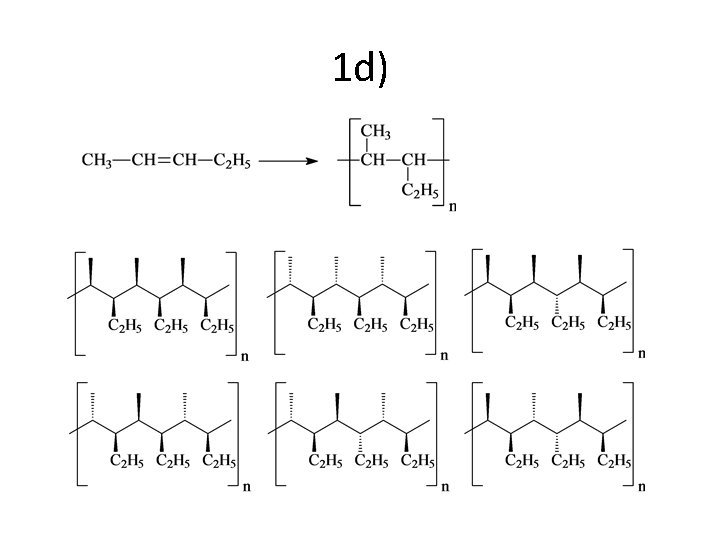
1 d)

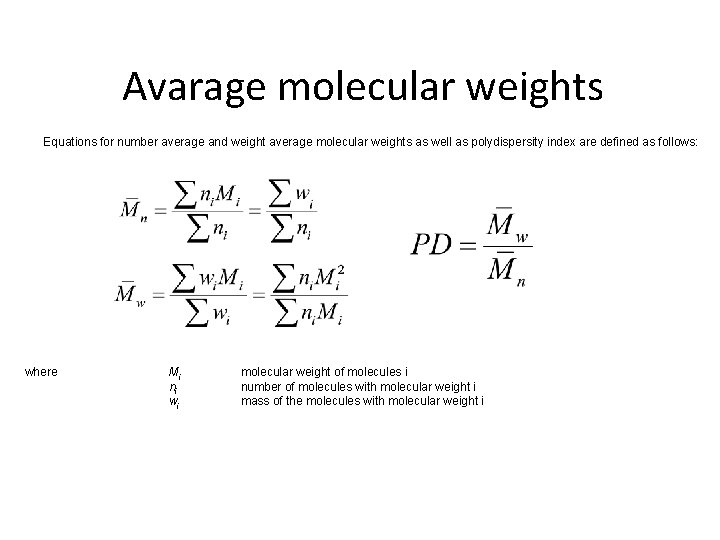
Avarage molecular weights Equations for number average and weight average molecular weights as well as polydispersity index are defined as follows: where Mi ni wi molecular weight of molecules i number of molecules with molecular weight i mass of the molecules with molecular weight i

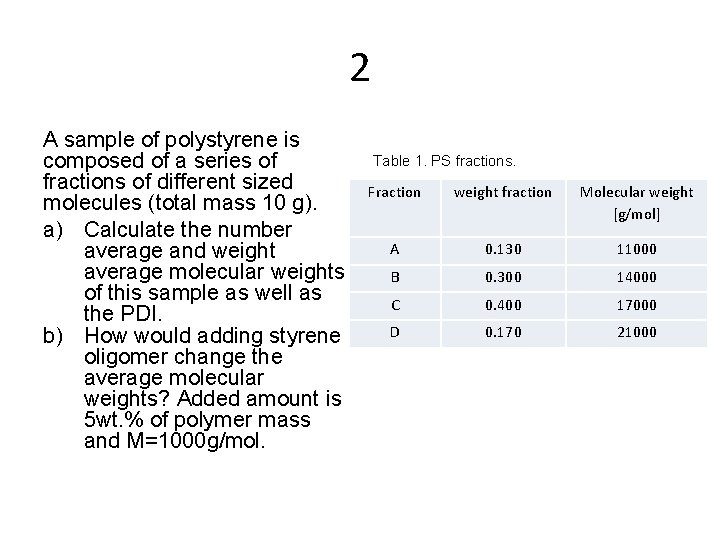
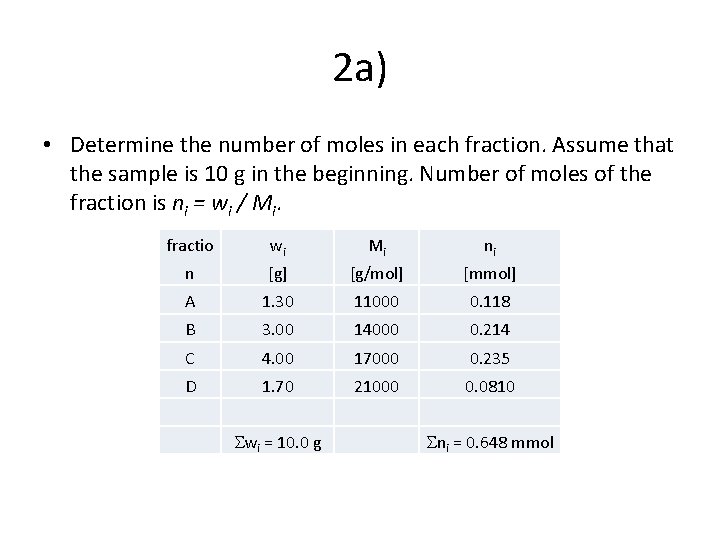
2 A sample of polystyrene is composed of a series of fractions of different sized molecules (total mass 10 g). a) Calculate the number average and weight average molecular weights of this sample as well as the PDI. b) How would adding styrene oligomer change the average molecular weights? Added amount is 5 wt. % of polymer mass and M=1000 g/mol. Table 1. PS fractions. Fraction weight fraction Molecular weight [g/mol] A 0. 130 11000 B 0. 300 14000 C 0. 400 17000 D 0. 170 21000

2 a) • Determine the number of moles in each fraction. Assume that the sample is 10 g in the beginning. Number of moles of the fraction is ni = wi / Mi. fractio wi Mi ni n [g] [g/mol] [mmol] A 1. 30 11000 0. 118 B 3. 00 14000 0. 214 C 4. 00 17000 0. 235 D 1. 70 21000 0. 0810 wi = 10. 0 g ni = 0. 648 mmol

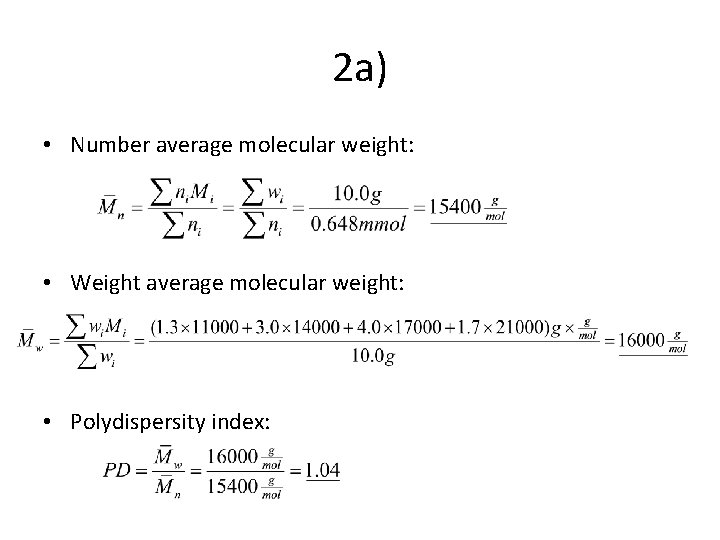
2 a) • Number average molecular weight: • Weight average molecular weight: • Polydispersity index:

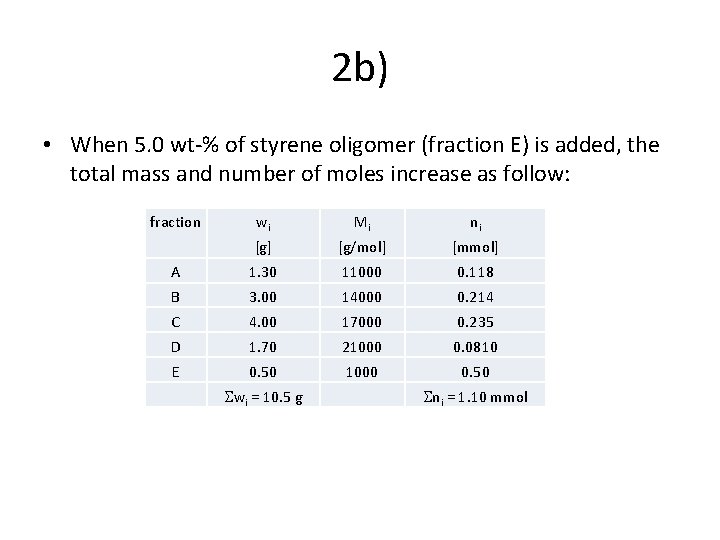
2 b) • When 5. 0 wt-% of styrene oligomer (fraction E) is added, the total mass and number of moles increase as follow: fraction wi Mi ni [g] [g/mol] [mmol] A 1. 30 11000 0. 118 B 3. 00 14000 0. 214 C 4. 00 17000 0. 235 D 1. 70 21000 0. 0810 E 0. 50 1000 0. 50 wi = 10. 5 g ni = 1. 10 mmol

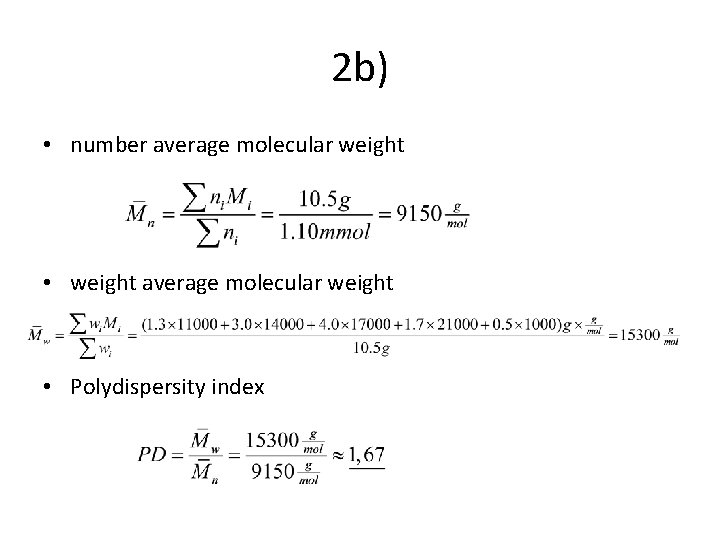
2 b) • number average molecular weight • weight average molecular weight • Polydispersity index

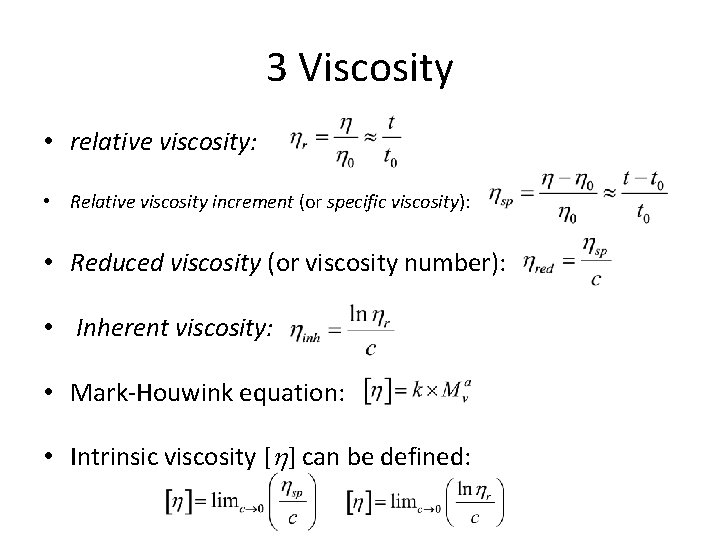
3 Viscosity • relative viscosity: • Relative viscosity increment (or specific viscosity): • Reduced viscosity (or viscosity number): • Inherent viscosity: • Mark-Houwink equation: • Intrinsic viscosity [ ] can be defined:

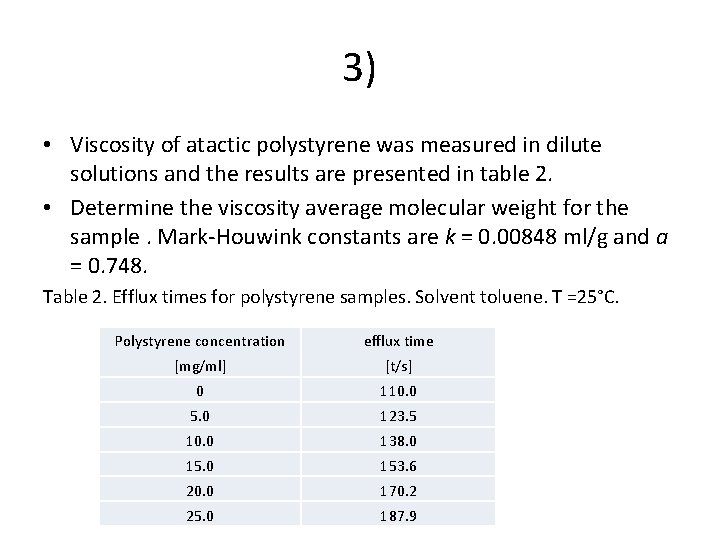
3) • Viscosity of atactic polystyrene was measured in dilute solutions and the results are presented in table 2. • Determine the viscosity average molecular weight for the sample. Mark-Houwink constants are k = 0. 00848 ml/g and a = 0. 748. Table 2. Efflux times for polystyrene samples. Solvent toluene. T =25°C. Polystyrene concentration efflux time [mg/ml] [t/s] 0 110. 0 5. 0 123. 5 10. 0 138. 0 153. 6 20. 0 170. 2 25. 0 187. 9
![3 Calculate the required viscosity parameters c efflux time mgml ts r 3) • Calculate the required viscosity parameters: c efflux time [mg/ml] [t/s] r =](https://slidetodoc.com/presentation_image_h/89342a01de98935b7987db60dbe220cd/image-15.jpg)
3) • Calculate the required viscosity parameters: c efflux time [mg/ml] [t/s] r = t/t 0 sp inh red = (t-t 0)/t 0 =ln( r)/c = sp/c 0 110. 0 5. 0 123. 5 1. 123 0. 0232 0. 0246 10. 0 138. 0 1. 255 0. 0227 0. 0255 15. 0 153. 6 1. 396 0. 0222 0. 0264 20. 0 170. 2 1. 547 0. 0218 0. 0274 25. 0 187. 9 1. 708 0. 0214 0. 0283 • Draw inh and red as function of concentration.
![3 is obtained from the plot from the crossing of yaxis 3) • [ ] is obtained from the plot from the crossing of y-axis:](https://slidetodoc.com/presentation_image_h/89342a01de98935b7987db60dbe220cd/image-16.jpg)
3) • [ ] is obtained from the plot from the crossing of y-axis: • and the average from these is [ ] = 23. 65 ml/g. Viscosity average molecular weight from Mark-Houwink equation: • Note! Due to empirical coefficients k ja a. the equation gives the molecular weight without unit. In literature k = 0. 007… 0. 01 and a = 0. 69… 0. 78 accuracy of the calculation is not particularly good.

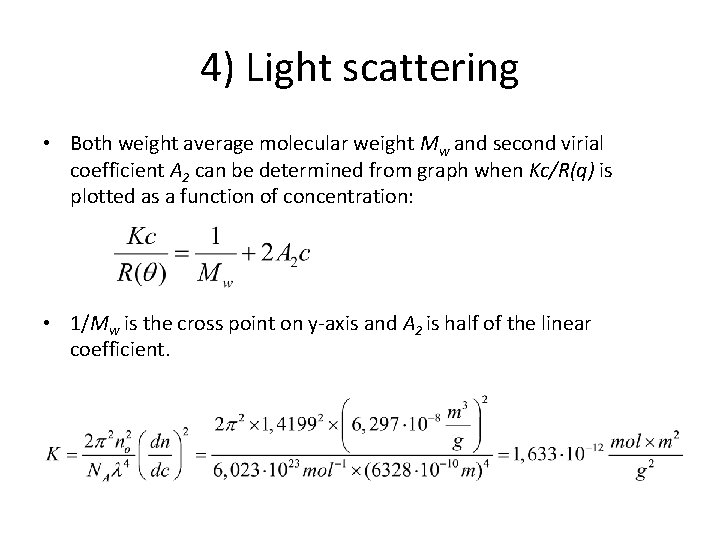
4) Light scattering • Both weight average molecular weight Mw and second virial coefficient A 2 can be determined from graph when Kc/R(q) is plotted as a function of concentration: • 1/Mw is the cross point on y-axis and A 2 is half of the linear coefficient.

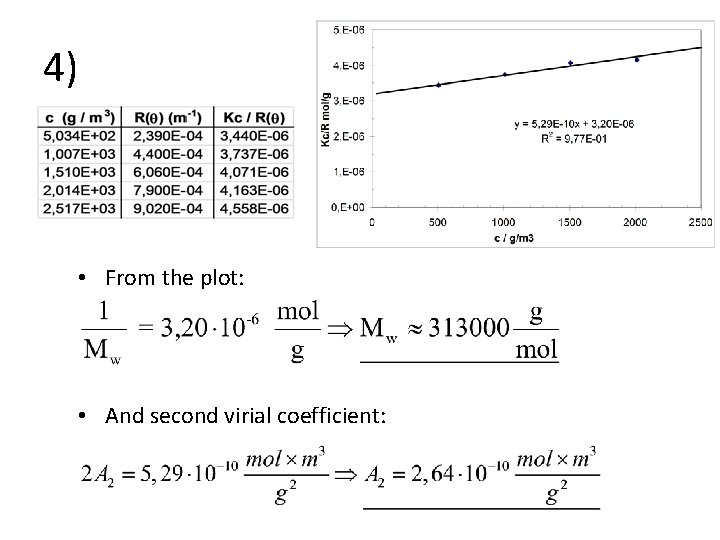
4) • From the plot: • And second virial coefficient: