Structure of Polymer Polymer Structure terms configuration and



- Slides: 27

Structure of Polymer

Polymer Structure • terms configuration and conformation are used to describe the geometric structure of a polymer • Configuration refers to the order that is determined by chemical bonds it is cannot be altered unless chemical bonds are broken and reformed • Conformation refers to order that arises from the rotation of molecules about the single bonds

Polymer Structure Conformation Cis Configuration Trans

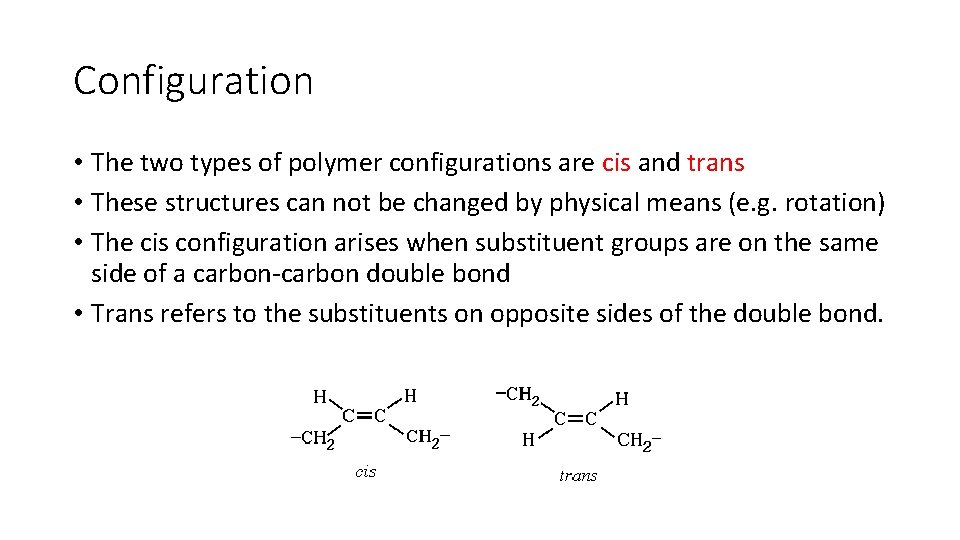
Configuration • The two types of polymer configurations are cis and trans • These structures can not be changed by physical means (e. g. rotation) • The cis configuration arises when substituent groups are on the same side of a carbon-carbon double bond • Trans refers to the substituents on opposite sides of the double bond.

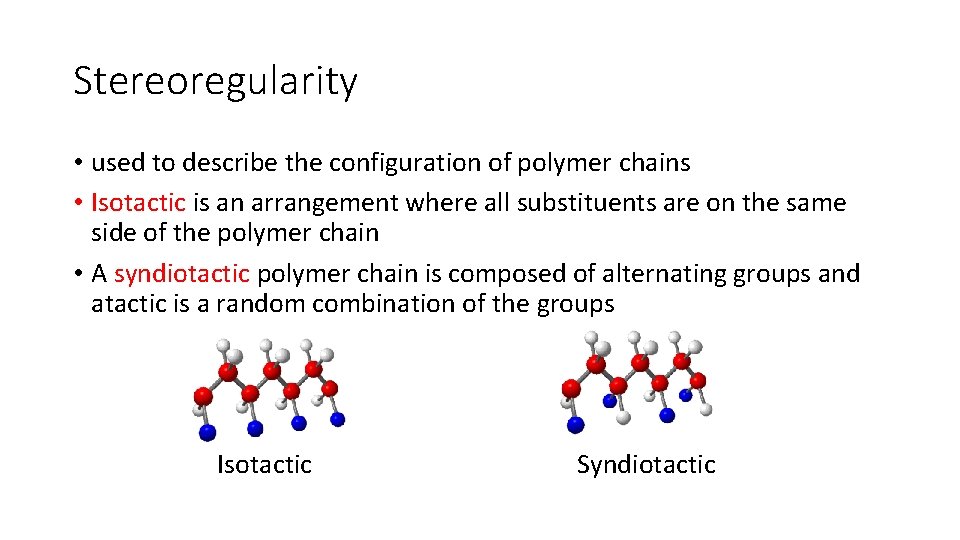
Stereoregularity • used to describe the configuration of polymer chains • Isotactic is an arrangement where all substituents are on the same side of the polymer chain • A syndiotactic polymer chain is composed of alternating groups and atactic is a random combination of the groups Isotactic Syndiotactic

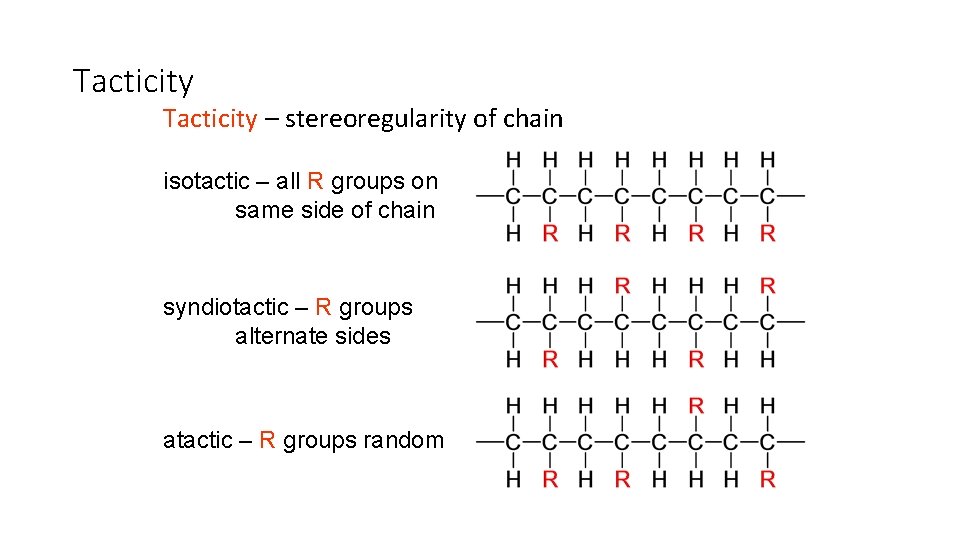
Tacticity – stereoregularity of chain isotactic – all R groups on same side of chain syndiotactic – R groups alternate sides atactic – R groups random

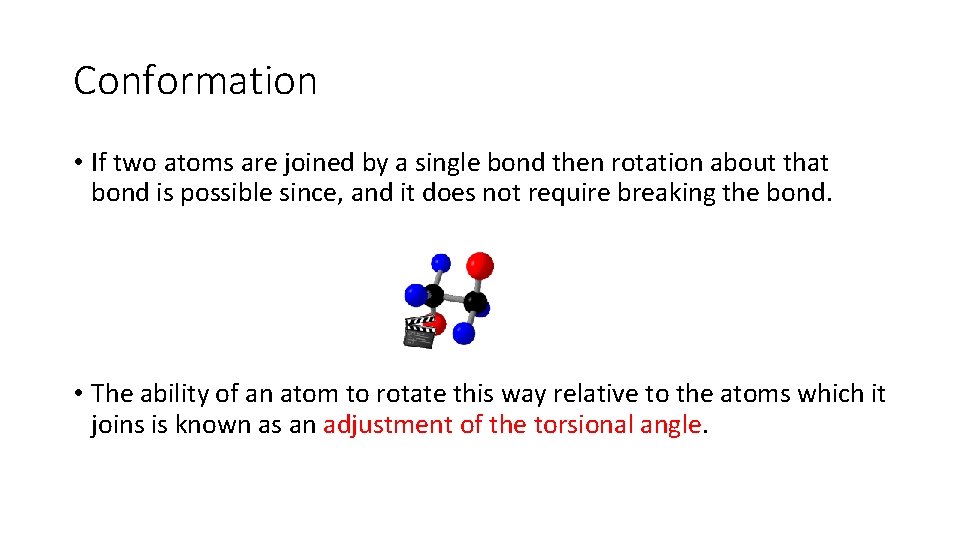
Conformation • If two atoms are joined by a single bond then rotation about that bond is possible since, and it does not require breaking the bond. • The ability of an atom to rotate this way relative to the atoms which it joins is known as an adjustment of the torsional angle.

A separate kind of chain structure arises when more that one type of monomer is involved in the synthesis reaction. These polymers that incorporate more than one kind of monomer into their chain are called copolymers.

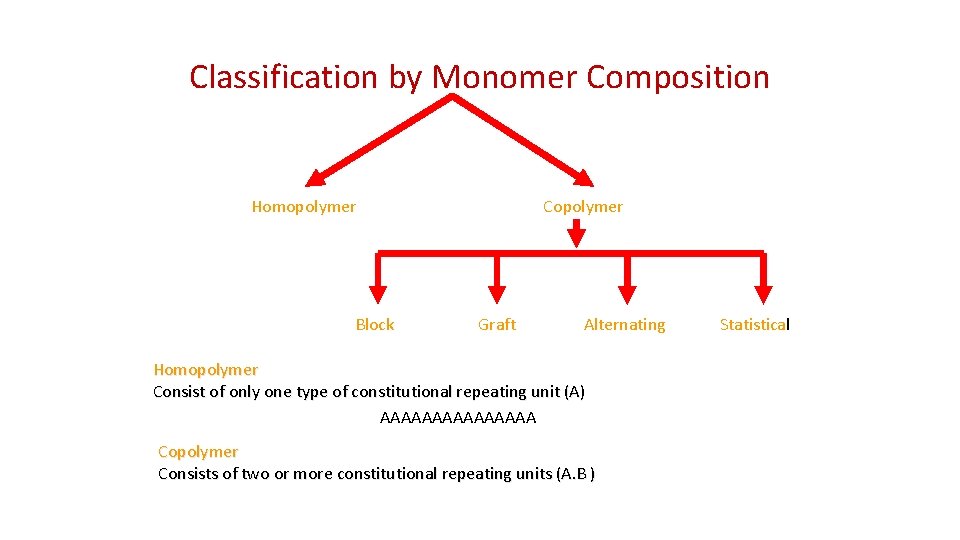
Classification by Monomer Composition Homopolymer Block Copolymer Graft Alternating Homopolymer Consist of only one type of constitutional repeating unit (A) AAAAAAAA Copolymer Consists of two or more constitutional repeating units (A. B ) Statistical

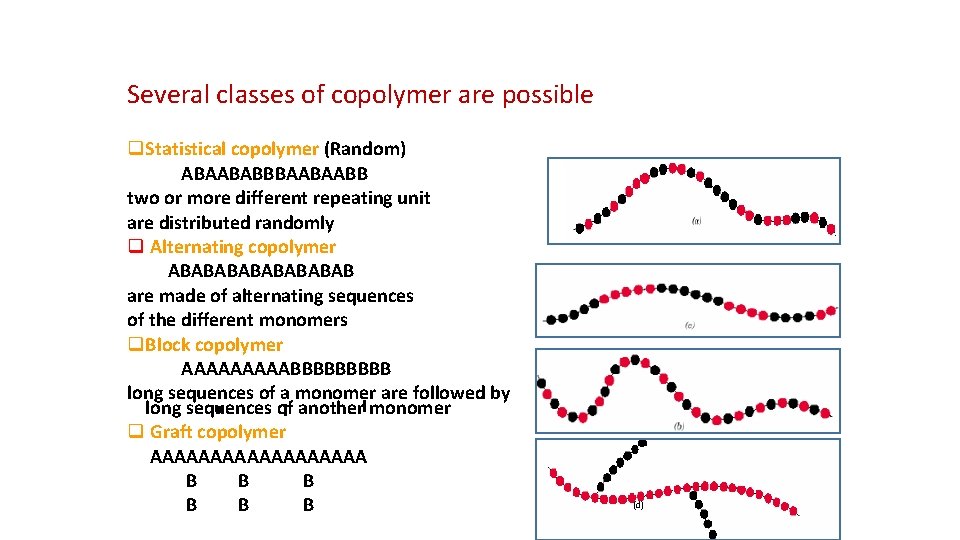
Several classes of copolymer are possible q. Statistical copolymer (Random) ABAABABBBAABAABB two or more different repeating unit are distributed randomly q Alternating copolymer ABABABAB are made of alternating sequences of the different monomers q. Block copolymer AAAAABBBBB long sequences of a monomer are followed by long sequences of another monomer q Graft copolymer AAAAAAAAA B B B (d)

POLYMERIZATION AND STRUCTURE OF POLYMERS

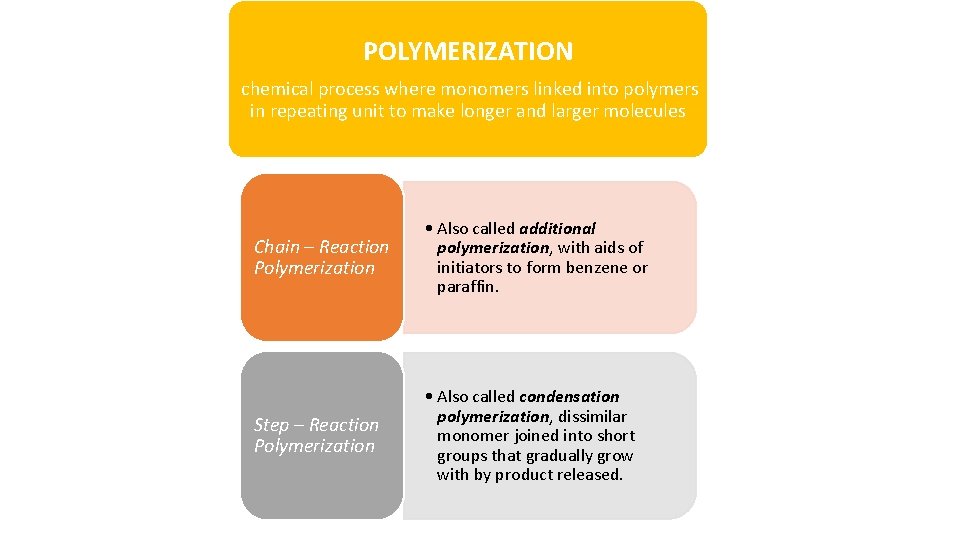
POLYMERIZATION chemical process where monomers linked into polymers in repeating unit to make longer and larger molecules Chain – Reaction Polymerization • Also called additional polymerization, with aids of initiators to form benzene or paraffin. Step – Reaction Polymerization • Also called condensation polymerization, dissimilar monomer joined into short groups that gradually grow with by product released.

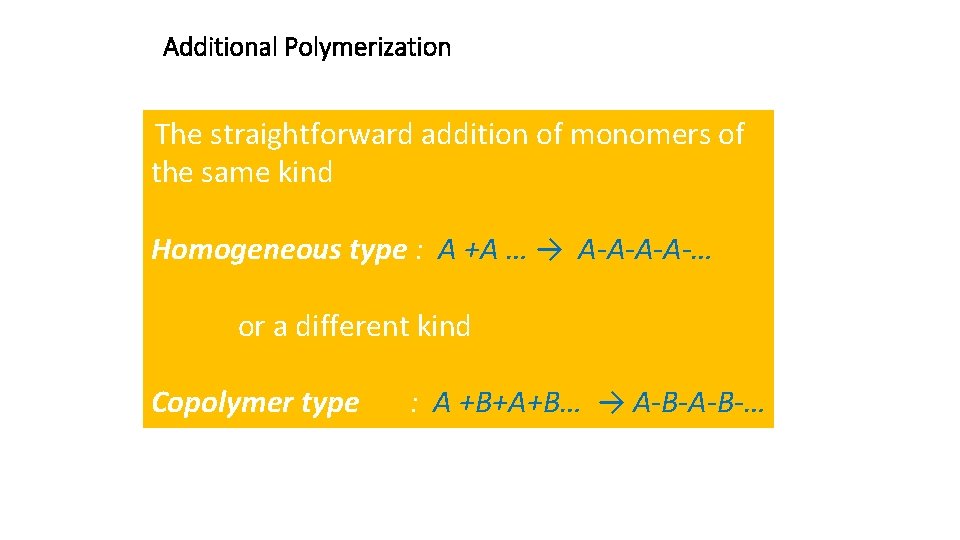
Additional Polymerization The straightforward addition of monomers of the same kind Homogeneous type : A +A … → A-A-… or a different kind Copolymer type : A +B+A+B… → A-B-…

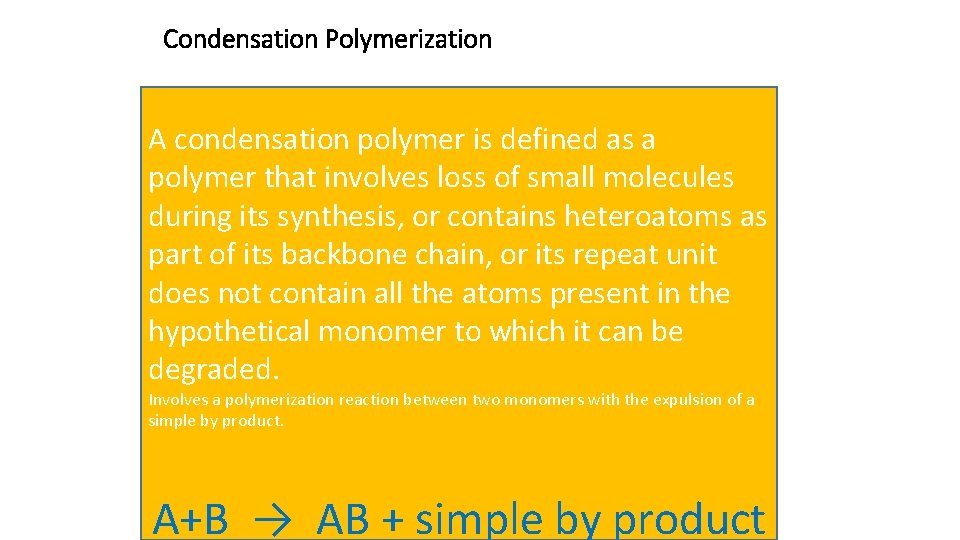
Condensation Polymerization A condensation polymer is defined as a polymer that involves loss of small molecules during its synthesis, or contains heteroatoms as part of its backbone chain, or its repeat unit does not contain all the atoms present in the hypothetical monomer to which it can be degraded. Involves a polymerization reaction between two monomers with the expulsion of a simple by product. A+B → AB + simple by product

Polymerization mechanisms - Step-growth polymerization

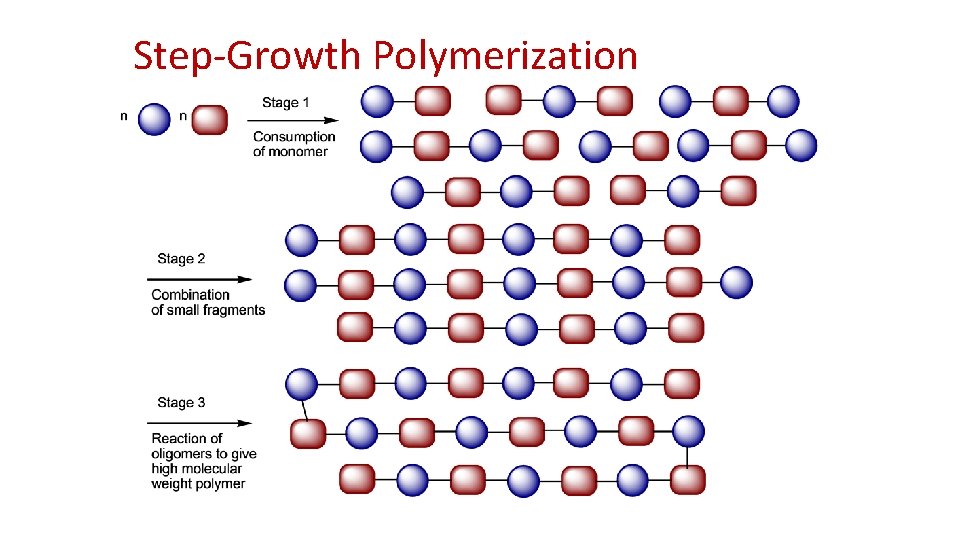
Step-Growth Polymerization

Step-growth polymers • formed by the stepwise reaction between functional groups of monomers • usually containing heteroatoms such as nitrogen or oxygen • Most step-growth polymers are also classified as condensation polymers but not all step-growth polymers release condensates • Step-growth polymers increase in molecular weight at a very slow rate at lower conversions and reach moderately high molecular weights only at very high conversion

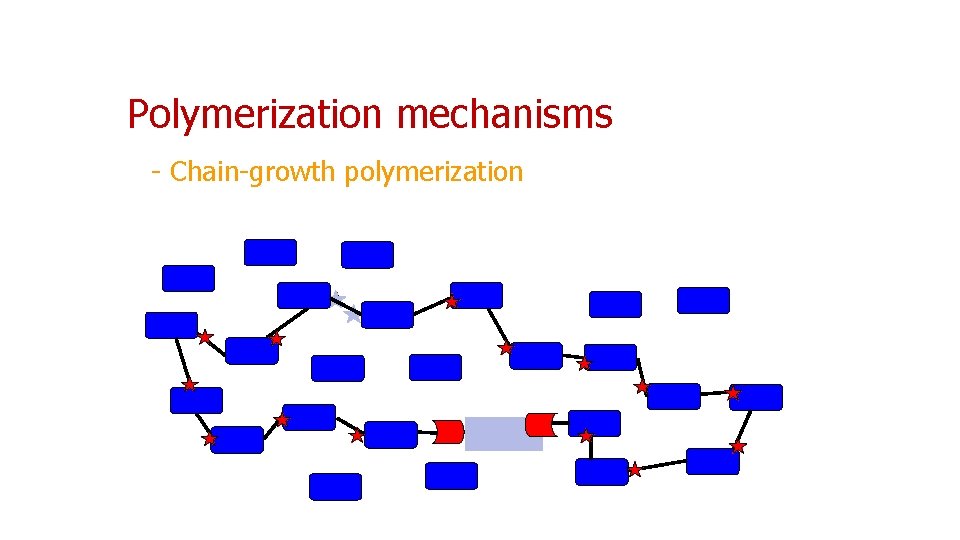
Polymerization mechanisms - Chain-growth polymerization

Chain-growth polymerization (or addition polymerization) • linking together of molecules incorporating double or triple carbon bonds • These unsaturated monomers (the identical molecules that make up the polymers) have extra internal bonds that are able to break and link up with other monomers to form a repeating chain, whose backbone typically contains only carbon atoms • Chain-growth polymerization is involved in the manufacture of polymers such as polyethylene, polypropylene, and polyvinyl chloride

Polymer Degradation • Polymer degradation is a change in the properties – tensile strength, colour, shape, etc of a polymer or polymer based product under the influence of one or more environmental factors such as heat, light or chemicals. • The term 'biodegradation' is limited to the description of chemical processes (chemical changes that alter either the molecular weight or solubility of the polymer)

Degradation: • CHEMICAL DEGRADATION • BIOLOGICAL DEGRADATION • MECHANICAL DEGRADATION • CHLORINE INDUCED CRACKING • THERMAL DEGRADATION • PHOTO DEGRADATION

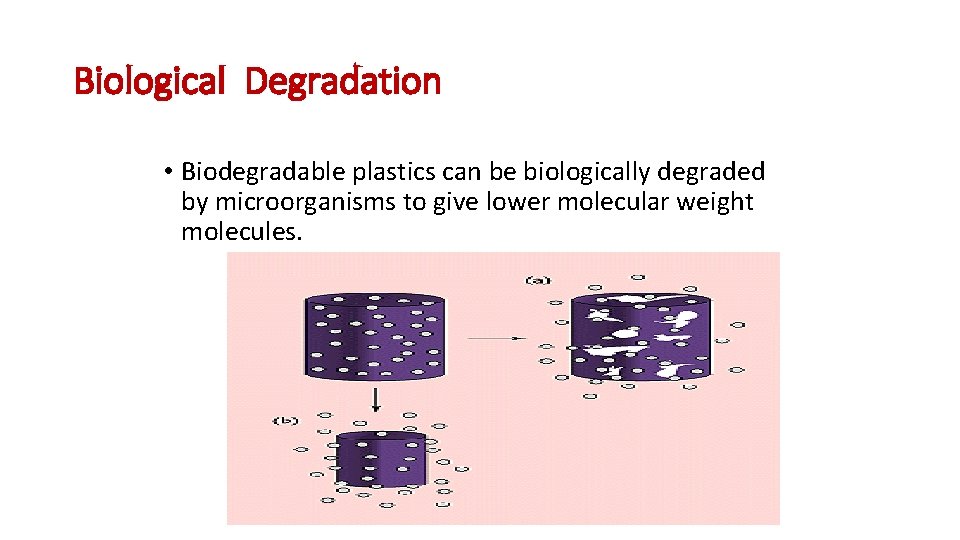
Biological Degradation • Biodegradable plastics can be biologically degraded by microorganisms to give lower molecular weight molecules.

Chlorine induced cracking • Another highly reactive gas is chlorine, which will attack susceptible polymers such as acetal resin and polybutylene pipe work. • There have been many examples of such pipes and acetal fittings failing in properties in the US as a result of chlorine-induced cracking.

Chemical Degradation: • Polymers can be degraded by solvolysis and mainly hydrolysis to give lower molecular weight molecules. • The hydrolysis takes place in the presence of water containing an acid or base. • Polymers are susceptible to attack by atmospheric oxygen, especially at elevated temperatures encountered during processing to shape.

Thermal degradation • Thermal degradation of polymers is molecular deterioration as a result of overheating. • At high temperatures the components of the long chain backbone of the polymer can begin to separate (molecular scission) and react with one another to change the properties of the polymer. • The chemical reactions involved in thermal degradation lead to physical and optical property changes relative to the initially specified properties.

Photo degradation • One of the disadvantages of using polymers in high temperature conditions or in outdoor applications – degradation • environment negatively influences the service life. • This process is called weathering - ageing an irreversible chemical process, • undesired changes of properties of the polymers, • discoloration and loss of mechanical properties

THANKS